QEEG Analysis and Applications
Introduction to the Quantitative Analysis of the Electroencephalogram
The history of recording the electrical activity of the EEG from animals dates back to Richard Caton in 1875, with the recording of human EEG not occurring until 1924 when Hans Berger recorded the first known human electroencephalogram (Britton et al., 2016). Graphic © Zayabich/Shutterstock.com.

The electroencephalogram has several unique advantages among the many imaging methods available for identifying and tracking information from the human brain and central nervous system.

The first is that it has excellent temporal resolution, which means it can show activity in the central nervous system quite quickly, in the range of 1 to 2 tenths of a second (100-200 ms). Compared to CT, MRI, fMRI, SPECT, PET, and other scanning methods, which often take several seconds to minutes for changes in the cerebral blood flow to be measured and recorded by the equipment, the electrical activity of the brain can be seen almost instantaneously with EEG. MRI graphic © Peastock/Shutterstock.com.
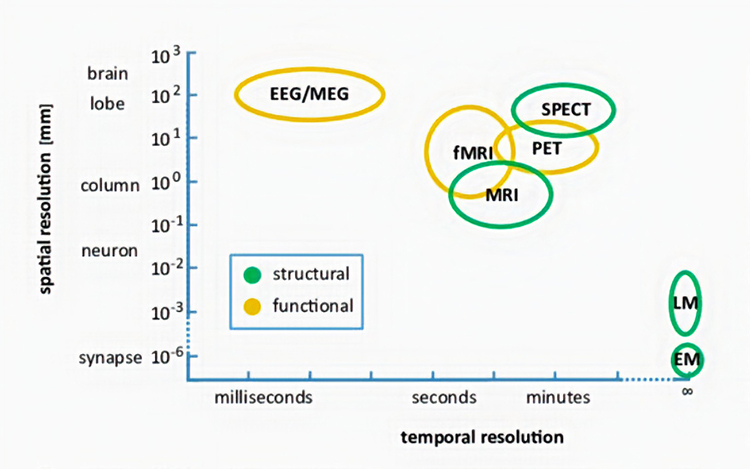
Each functional neuroimaging method can be rated with respect to how quickly it can detect changes in function (temporal resolution) and over how small an area it detects changes in function (spatial resolution). Whereas EEG and MEG methods detect changes in function most quickly, they are less able to detect the precise area where functional changes occur compared to fMRI. Graphic from Pfister et al. (2012) © Mathematics and Visualization. EM = electron microscope and LM = light microscope.
Another benefit of the electroencephalogram is the ability to identify active communication pathways. Diffusion tensor imaging (DTI) can show the physiological pathways of the brain in amazing detail and can show physical damage to these connecting fibers as well. Graphic is courtesy of NeuroGuide's TM NeuroNavigator.
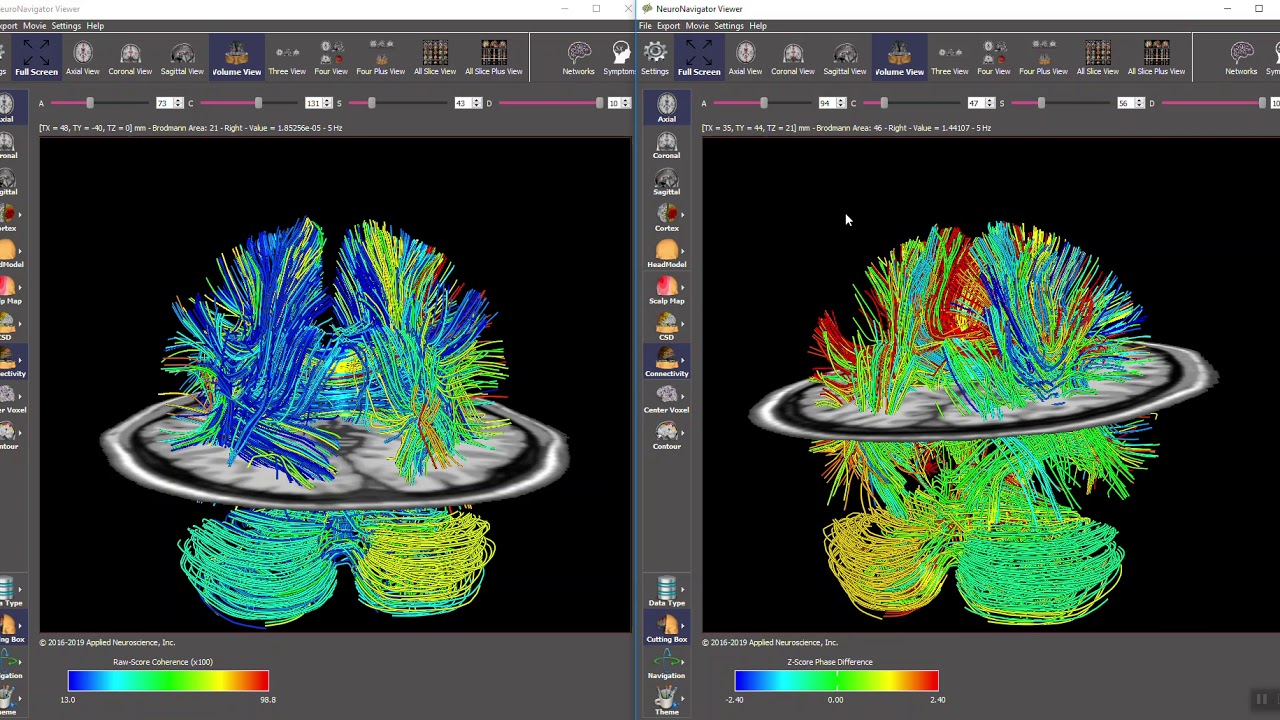
Caption: Raw scores and Z-Scores in Diffusion Tensor Imaging (DTI) Fiber Bundles.
However, it cannot determine whether these pathways carry communication from one area to another efficiently and appropriately. Through measurements of coherence, phase, phase slope index, and other metrics, the electroencephalograph can provide a window into the communication characteristics of an individual brain and identify both appropriate function and deviations from that desired function.
Finally, the EEG can identify areas functioning differently than expected through quantitative EEG imaging, also known as qEEG. Often these areas of apparent dysregulation represent damage due to injury, possible developmental delay or even dysfunctional activation patterns resulting from life experiences and emotional and psychological trauma.
The progression from recording an EEG to the quantitative analysis of this information requires the same care and concern needed for a typical clinical electroencephalogram recording. Attention to correct sensor placement, quality of connection, signal quality, and the presence of artifact (electrical activity), either physiological or exogenous (coming from outside the body), are all vitally important. Additionally, the inspection of the resulting EEG recording and the accurate selection of EEG activity and rejection of electrical activity that reflects anything else is of great importance so that the resulting digital and quantitative analysis can be as accurate as possible.
International QEEG Certification Board Blueprint Coverage
This unit covers VII. QEEG Analysis & Neurofeedback Application (6 hours).

This unit reviews EEG Recording and Data Analysis (Recording the Data, Reducing Artifacts, Visual Inspection of Session Data, EEG and qEEG Refresher, EEG Activity Is Absorbed and Smeared By Tissues, Amplitude Refresher, Frequency Refresher, EEG Source Refresher) and Client Training (SMR Training Example, The Case for Multiple Approaches, Symptom Matching Approach, qEEG-Guided Approach).
EEG Recording and Data Analysis
In this section, we will discuss the requirements for a good EEG recording, the methods for accurately selecting good quality EEG data, methods for rejecting artifacts, and the translation of the recorded EEG activity to imaging methods such as quantitative EEG database comparison and other types of analysis and interpretive software. We will also learn to interpret the resulting analyses from different software programs and learn the benefits and limitations of each approach.
This may seem like a somewhat daunting task and the study of the electroencephalogram is certainly one of lifelong learning. However, the beginning electroencephalographer can learn to identify good quality EEG as opposed to artifact and can learn to interpret the resulting analyses in a way that will allow them to assess their client and provide guidance for subsequent neurofeedback training.
Recording the Data
The initial EEG recording requires multiple components for a good recording experience. All of these elements should be in place before seating the client. Following are some tips and tricks from experienced electroencephalographers that will make the client experience more positive and, therefore, the quality of the recording more accurate.Preparing the client before even applying the electrode cap or the individual electrodes is important. Emphasize that the recording process is non-invasive and that nothing is being done to the client. Let the client know that the EEG recording device is simply sensing and recording the electrical activity produced by the brain and that there is no input from the device to the client's brain. For children, it can be useful to allow them to handle the sensor cap or individual sensors under direct supervision so they can become comfortable with these components. Finally, using the term sensors rather than electrodes is important, as people often have negative perceptions of the latter term.
Begin EEG analysis during the recording process. The recording position should be comfortable, and the client should be at a slight recline with feet elevated. This can be accomplished with a reclining chair, though large, overstuffed recliners are often not comfortable due to large 'pillow' sections that can push the head forward and cause increased artifacts. Some practitioners have reported that zero-gravity chair types are good choices. The recline should be no more than 30-45 degrees from upright. Too little recline can cause neck discomfort, and too much recline can encourage drowsiness.
A rolled towel can be used behind the neck to support the neck, keep posterior sensors away from the chair surface, and relieve EMG artifacts in the neck and head. A towel or absorbent pad should also be used behind the head to absorb any gel or paste that escapes the sensors to prevent bridging artifact (discussed elsewhere) and to protect the chair.
The recording environment should be quiet, and the lighting should be moderate. If lights are too bright, it can cause discomfort during the eyes-open portion of the recording, and if they are too dim, it may result in drowsiness. The eyes-open and eyes-closed segments should be done sequentially so that a comparison between the eyes-closed alpha response and the eyes-open alpha blocking may be observed in the recording. There should also not be any movement around the client, particularly during the eyes-open recording, that could distract the client and cause eye movement artifacts.
Reducing Artifacts
Visual inspection of the EEG during recording should guide efforts to reduce artifacts. This is best done during the recording rather than trying to remove artifacts after the fact. The movie below of bursting alpha shows the sequential synchronization/desynchronization of groups of neurons. Higher voltage bursts are followed by voltage decreasing toward zero. These voltage fluctuations reflect rhythmic changes in the local field potential. This BioTrace+ /NeXus-32 video © John S. Anderson.Instruct the client to clench teeth, frown, raise eyebrows, blink, move the eyes up and down, right and left, move the head side to side, up and down, and swallow to produce an artifact. Allow the client to view the artifact as it is happening so that they may see the effects and understand why it is important to reduce such movements to ensure quality recording. Have the client observe the recording screen in a relaxed state for approximately 1 minute and encourage them to reduce any remaining artifacts.
Most clinicians recording EEG for database comparison and/or other quantitative analysis will record each condition for between 5 and 10 minutes. That means 5-10 minutes for eyes open and the same for eyes closed, and then possibly some similar period for mental math exercises, reading tasks, and other tasks as desired. It can be quite helpful to record a sequence of 5 or 6 eyes-open and eyes-closed segments that each last from 15-30 seconds to understand better this client's alpha response and alpha blocking dynamics. Prompt the client to open or close the eyes at 15-30 second intervals. Be sure to mark the beginning and end of this sequence.
Suppose the client is anxious or physically tense or producing excessive artifact in the initial recording periods for eyes-open and eyes-closed conditions. In that case, recording another 5-10 minutes of each toward the end of the recording period may be helpful. At this point, the client is often less anxious because they have become familiar with the process and may show a more accurate representation of their typical EEG activity. Video © John S. Anderson.
Continue to observe both the client and the recording during the recording process to ensure that the client does not begin to produce increased tension, possibly due to an uncomfortable physical condition, a need to sneeze or cough or swallow, a need to use the restroom or any other source of discomfort. The recording can be paused in most cases. In some cases, the cap can be disconnected from the amplifier without causing any difficulty, but check with your manufacturer's instructions to determine whether this is so. Certainly, the client can be allowed to sneeze or cough, shift position, have a drink of water, or have other relief from difficulty.
The client should NOT observe the screen during the recording. If the client watches the screen, their eye movements will cause excessive artifacts in the recording.
Mark any events, such as the beginning of behavioral segments like eyes open and eyes closed, to facilitate post-recording assessment.
Mark behavioral conditions including: eyes open (EO), eyes closed (EC), mental math (MM), reading (RE), eyes-open/eyes-closed sequences (EO/EC), and any other tasks. Alternatively, separate sections can be saved for each condition. Choose the approach that is best for you and your software.
If excessive artifact is noted, pause or stop the recording and correct it before continuing. If the artifact is coming from the client, helping the client learn to relax and keep the eyes relaxed will help reduce the artifact. Many artifacts are subtle and may be difficult to observe in the client. Muscle clenching, chin thrusting or retracting, tongue movements, and others may not be obvious. Additionally, the lateral eye movement that occurs in most EEGs during the eyes-closed condition is difficult to see as the recording progresses and often requires that the clinician observe the client closely to see the movement of the eyes underneath the closed eyelids.
Clients are rarely consciously aware of this movement of the eyes, and even when asked to relax the eyes and stop moving them side to side, they will be unable to do so. As a solution, it is sometimes helpful to place large cotton balls over the closed eyelids so that the client may feel the movement of the eyes. This should be done with no pressure on the eyes. A couple of techniques can be used to accomplish this task. A loosely applied strip of paper tape from above the eye to below the eye on the cheek can keep the cotton ball in place, or a loosely fitting eye mask can also be used to keep the cotton balls in place. These are removed before other tasks are performed.
Visual Inspection of Session Data
The initial visual inspection can occur in the original recording software or an EEG analysis and database program (database for short). EEG database software often has many more analysis tools than all but the most sophisticated EEG recording programs and is usually a better choice for the visual analysis process. Import data into the EEG database using the appropriate method for your device or software, be sure to correctly indicate the client's date of birth and the eyes condition. Video © John S. Anderson.The preceding video has demonstrated the process of the initial visual inspection of the EEG using multiple montages to determine the information's accuracy and identify the artifact. Then some initial assumptions are made from the visual inspection that will be explored more fully using quantitative analysis. Following the visual inspection, select the best of the "artifact-free" segments. Then the process continues with the creation of topographic maps, tables, and other visual displays that provide useful views of the data using a variety of mathematical manipulations of the data.
Clearly, the client's EEG is simply a representation of the activity that occurs in the brain and the nervous system generally. Cortical neurons communicate and interact with multiple mechanisms throughout the body and brain and produce a variety of electrical and chemical behaviors that reflect all of this interactivity.
EEG and qEEG Refresher
Both the EEG and qEEG can be conceptualized as functional imaging techniques. A single-channel EEG performs “neuroimaging” by displaying an image of microvolts in adjacent 1-Hz bins or adjacent bands (e.g., a 2D spectrogram (shown below) or with a 3D spectrogram. Graphic © John S. Anderson.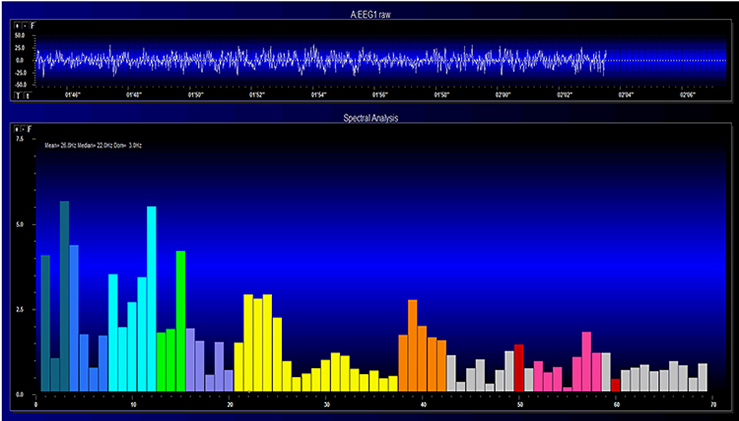
Further, 19-channel qEEG methods show images of activity as it is distributed across the brain’s convexity (i.e., over a 2D 10-20 map) or in three dimensions using more advanced qEEG methods (e.g., LORETA). Graphic courtesy of BrainMaster Technologies.
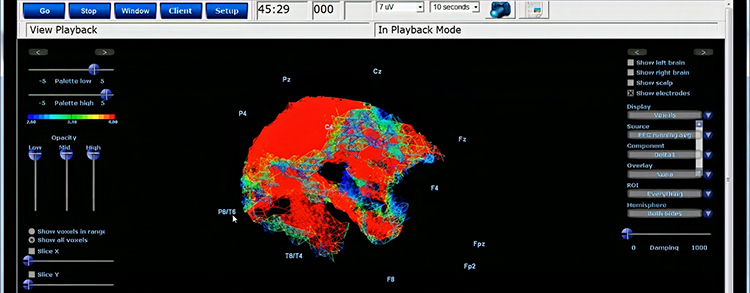
EEG Activity Is Absorbed and Smeared By Tissues
The electrical activity that reaches the scalp surface is significantly attenuated and mixed together by its passage through the various tissues and structures that lie between the brain and the scalp, including the pia mater, subarachnoid space, arachnoid matter, subdural space, meningeal and periosteal dura matter, bone of the skull, periosteum and the skin of the scalp.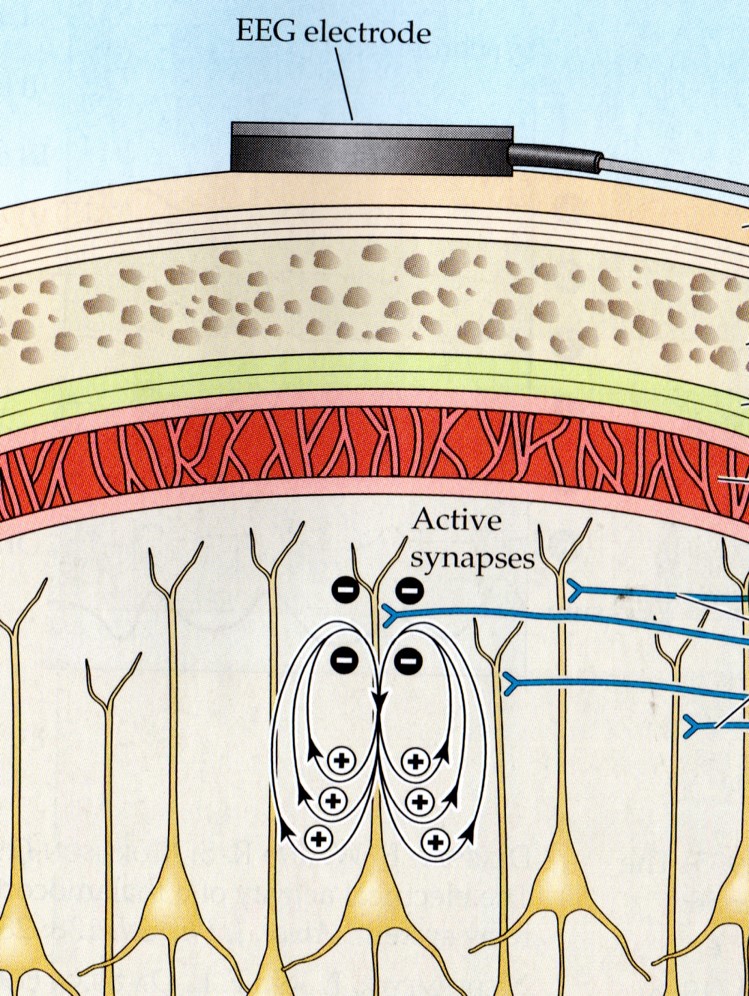
Each of these structures and tissues impedes the flow of electrical activity so that what begins as millivolt readings (thousandths of a volt), when recorded using intracortical electrodes, becomes microvolt readings (millionths of a volt) upon reaching the scalp surface. Graphic © Alilia Medical Media/Shutterstock.com.
One of the roles of the EEG is to separate the signal into its component parts, essentially amplitude and frequency.
Amplitude Refresher
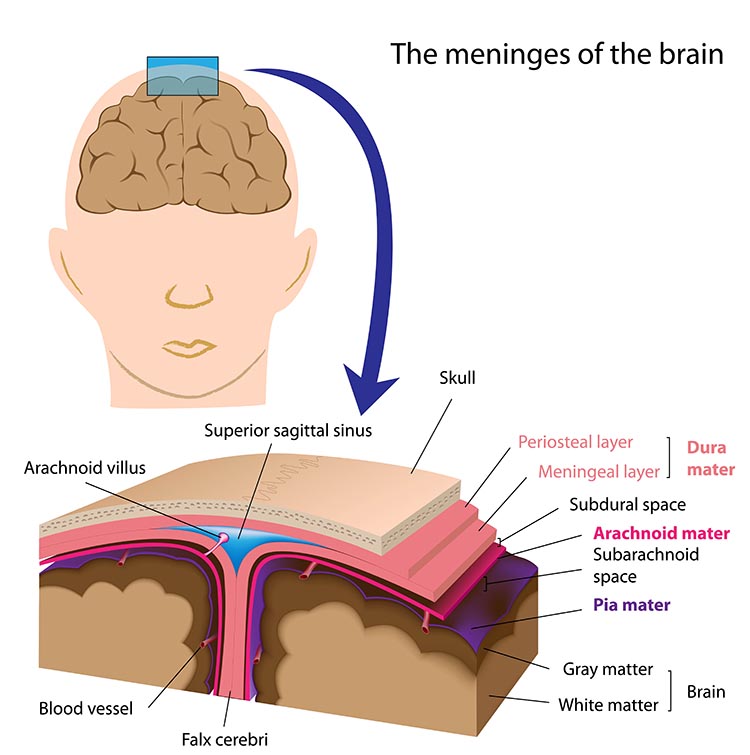
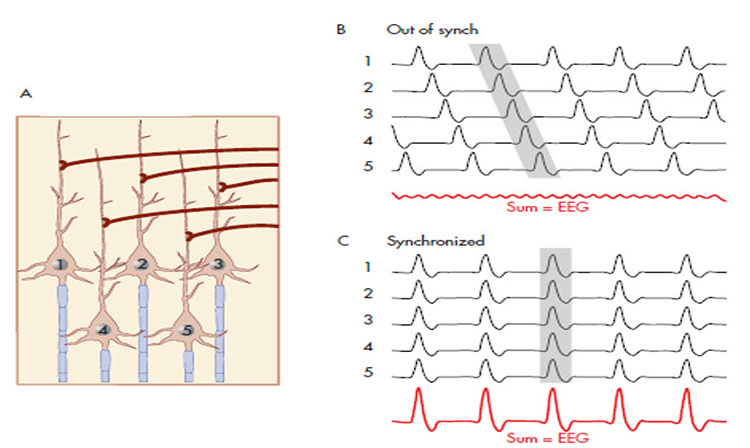
The "amount" or amplitude and the "pattern" or morphology of any EEG frequency band reflect the number of neurons discharging simultaneously at that frequency. Lower numbers of neurons firing synchronously correspond to lower signal amplitude.
Amplitude measures the amount of energy in the signal and is usually expressed in microvolts.
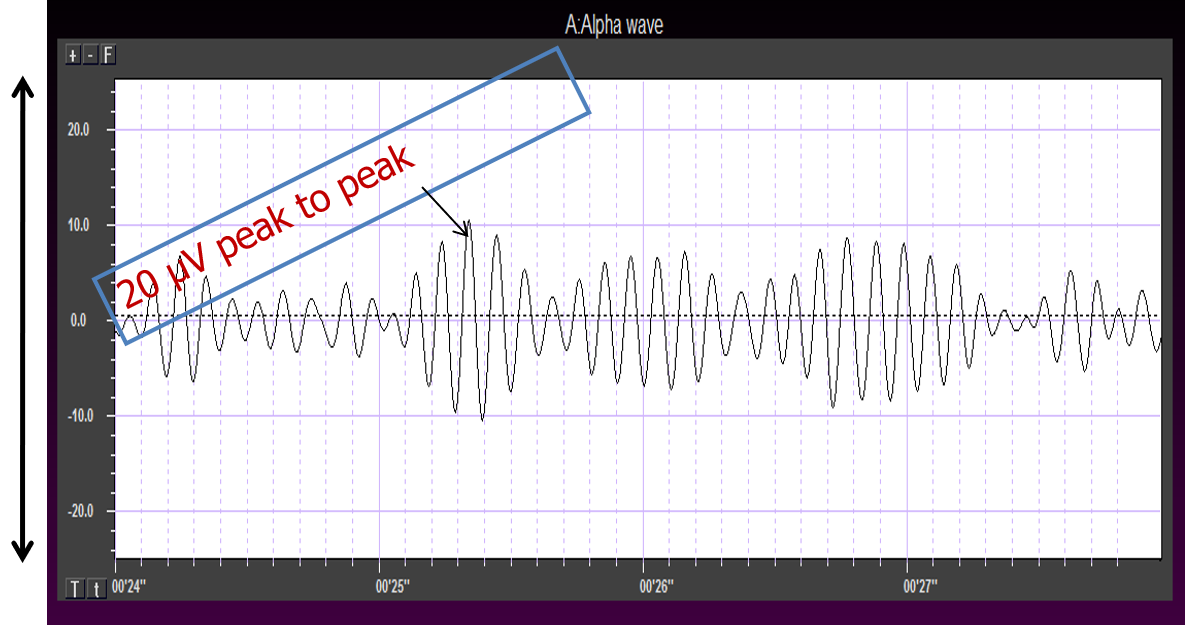
Greater synchrony in firing among neurons results in higher amplitude, as shown with alpha in the graphic below.
Greater firing synchrony produces larger EEG potentials that can be measured from the scalp surface.
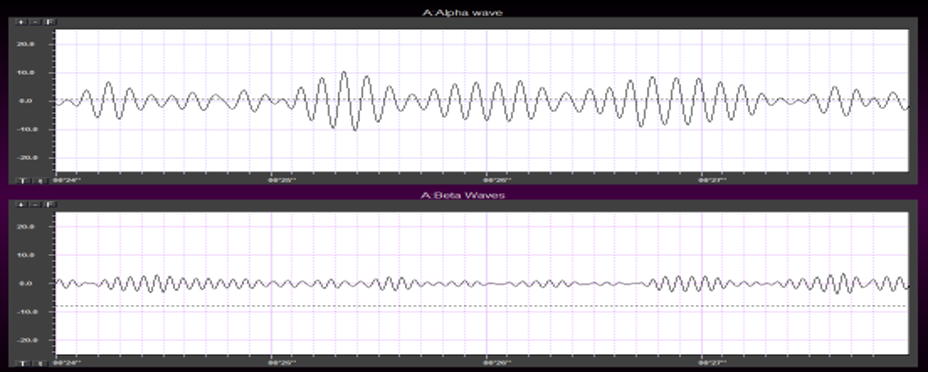
Frequency Refresher
The raw EEG contains all EEG frequencies, just as white light contains all light frequencies. Digital filters separate the EEG frequencies just as a prism separates individual colors. Graphic © kmls/ Shutterstock.com.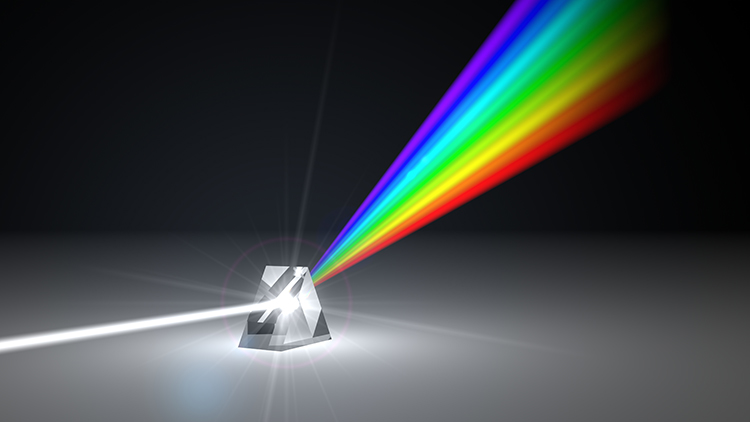
EEG frequency is measured in cycles per second or Hz. Count the number of peaks or count the number of zero (0.0) crossings divided by 2.
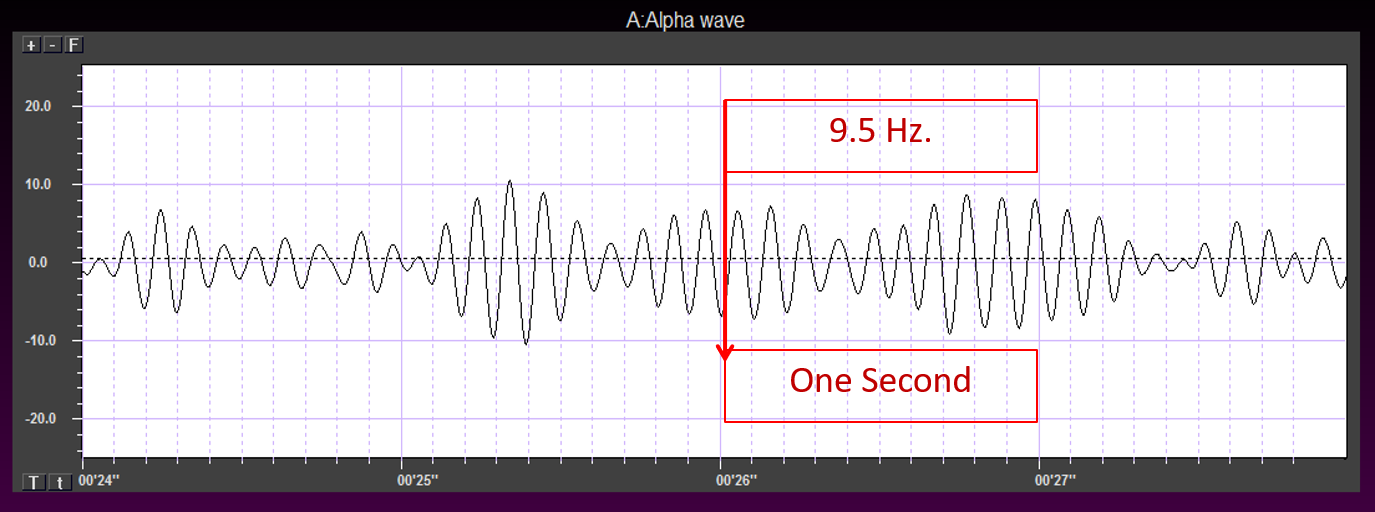
The slower the waves, the lower the EEG frequency.
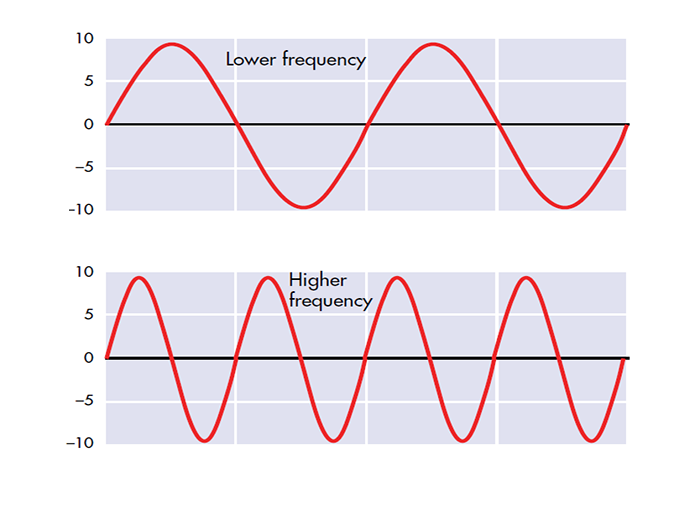
The longer the wavelength, the slower the frequency.
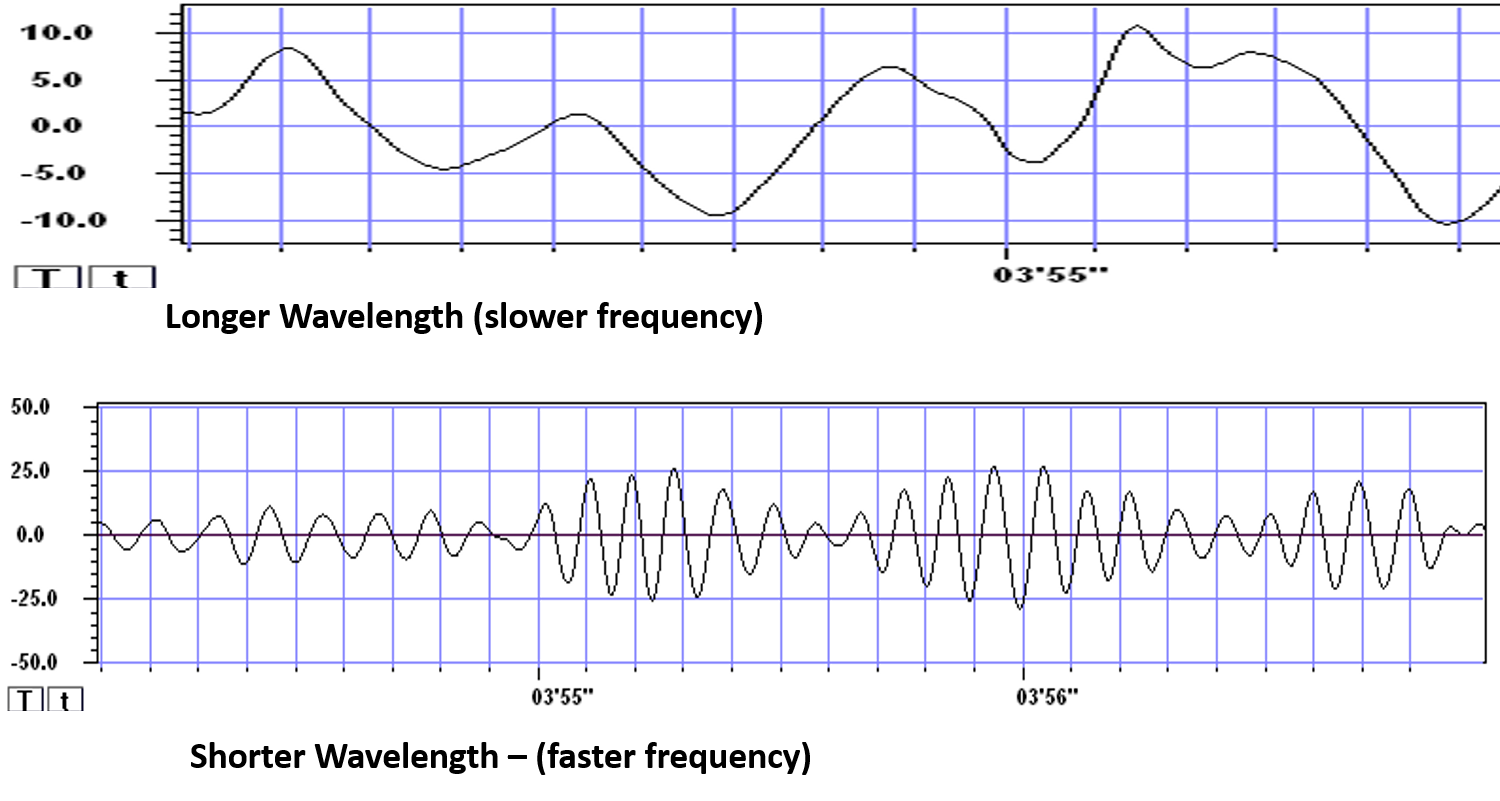
The movie is a single-channel BioTrace+ /NeXus-32 display of EEG activity from 1-64 Hz activity broken into its component delta, theta, alpha, and beta frequency bands by digital filters © John S. Anderson.
The wave patterns we see on our computer screens occur due to the methods we use to display the EEG data. The brain does not make "waves" as such. Neurons sometimes fire synchronously, and when we plot these electrical signals on a graph, they appear as waveforms (see graph below).
The two graphs below show the incoming EEG plotted as a series of dots representing the number of measurements of the amplitude or voltage of the EEG signal during a one-half-second (500-ms) period. The dots represent voltage, they are placed on the graph according to the y-scale or vertical axis that shows voltage differences from -1 to +1 (the value this represents is an arbitrary measure).
Each time a sample is made, it is plotted on the graph. Once the samples are plotted, and a line is drawn between them, the waveform appears. In most cases in real EEG plotting, there are so many samples per second that the dots appear to create a solid line. This graphic shows the individual dots as distinct points for clarity. Note that a greater number of dots or samples per second (SPS) results in a more clear and more accurate representation of the signal.
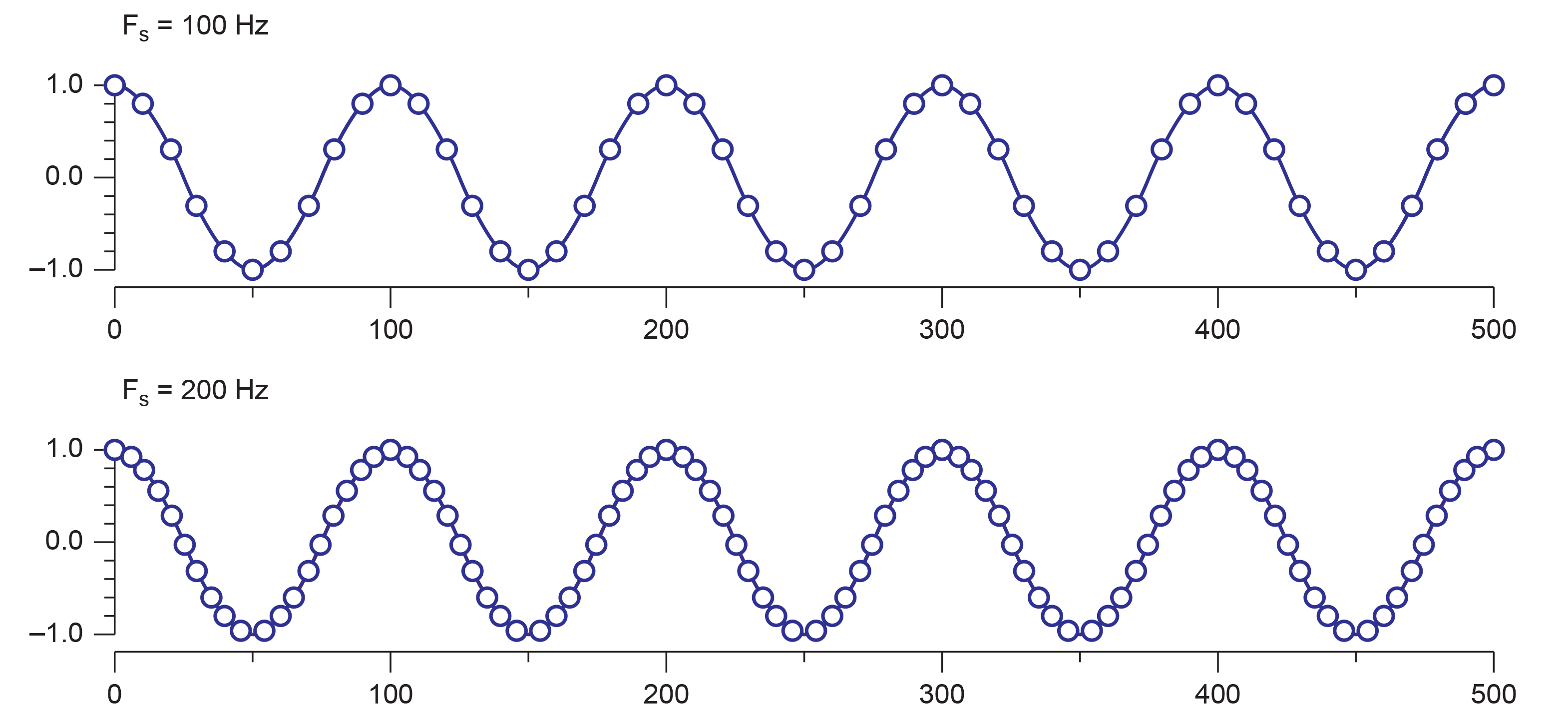
The graphs below make this difference even more obvious. The graphs show the same EEG recording signal, time (10 seconds), and amplitude (100 uV, i.e., -50 to +50 uV) scale. The top graph was sampled at 64 samples per second SPS) for an EEG frequency band of 1-45 Hz. The bottom graph sampled the same 1-45 Hz signal at 256 SPS. The differences in detail and level of information available with the faster sample rate (more frequent samples each second) are clearly apparent. A more in-depth discussion of these principles appears in qEEG Tutor's Recording and Editing the Raw EEG unit.
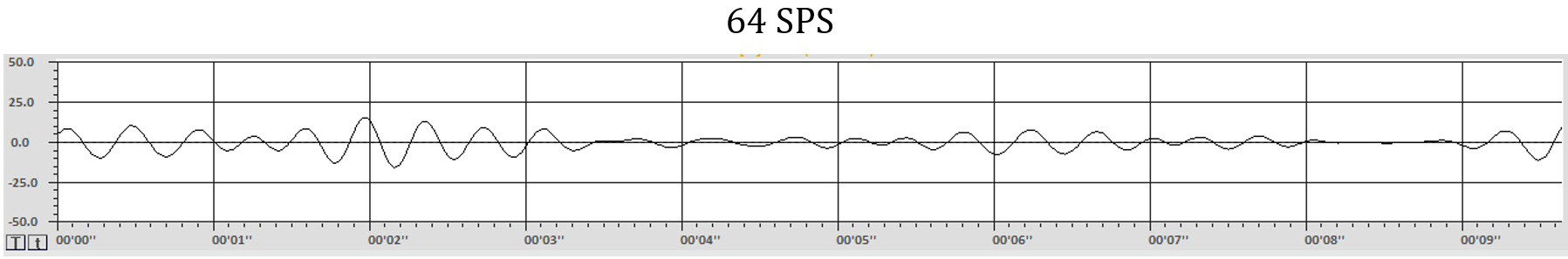
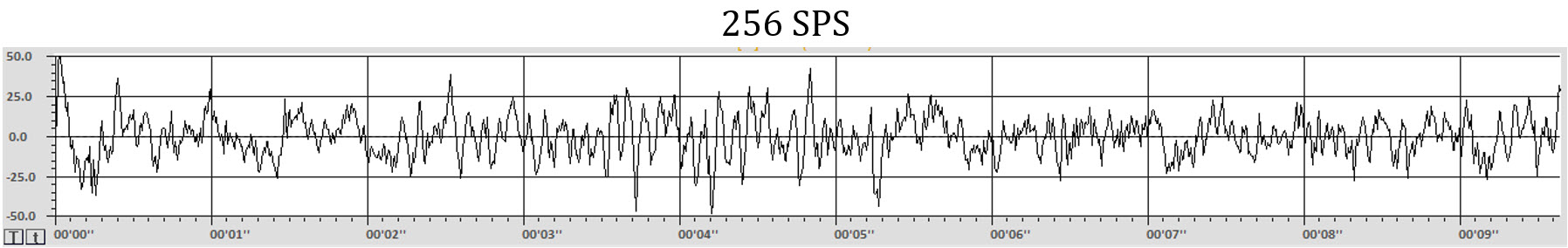
The appearance of the waveforms of the EEG represents multiple factors, including amplifier characteristics such as the bit-size of the analog-to-digital converter, degree of filtering, sample rate both when the data is collected and in the software, even the resolution of the computer monitor upon which the data is displayed and more.
It is clear that the EEG we observe is a construction created by humans and only represents the electrical activity of the brain and nervous system. It is similar to how a photograph of a chair represents the chair but is not actually the chair and is a pale representation of the real physical object. However, the EEG gives us a window into the nervous system's activity. Through repetition and experience, we can use it to begin to identify important characteristics of that nervous system.
EEG Source Refresher
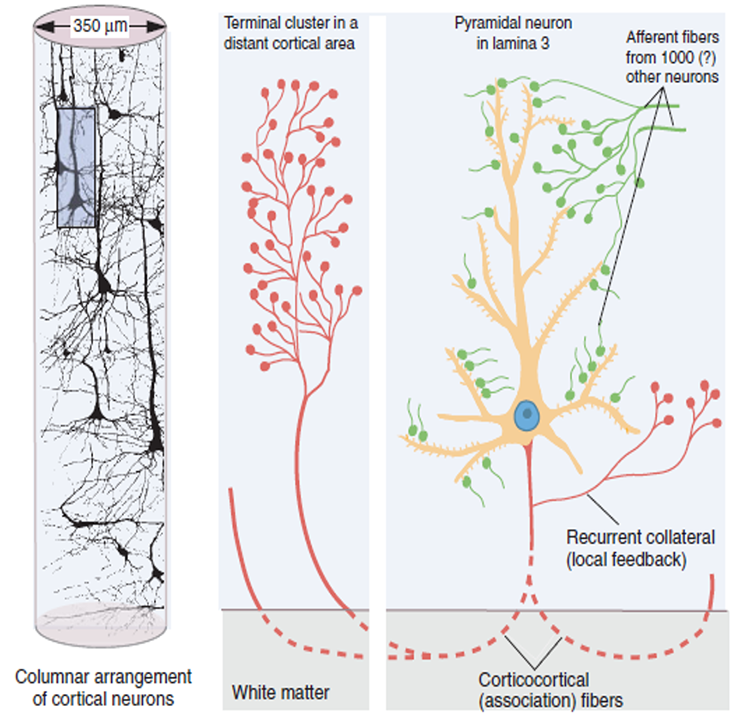
The scalp EEG results from the summation of large areas of gray matter activity. Areas are polarized synchronously due to the input of oscillatory or transient evoked activity. These areas comprise thousands of cortical columns containing large pyramidal cells aligned perpendicularly to the cortical surface.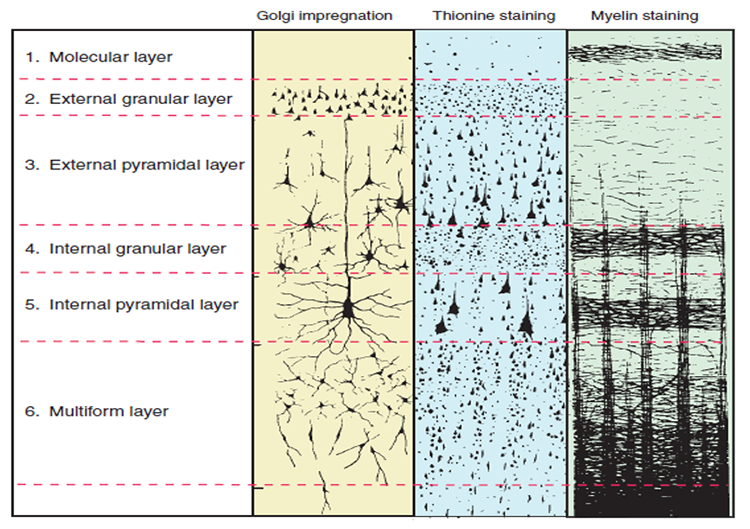
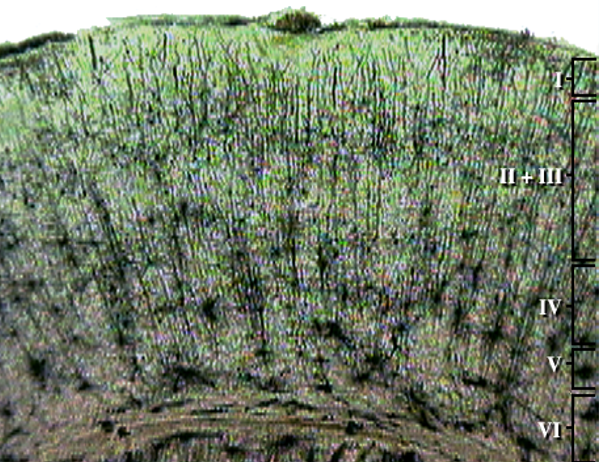
Pyramidal neurons are found in all cortical layers, except layer 1, and represent the primary type of output neuron in the cerebral cortex.
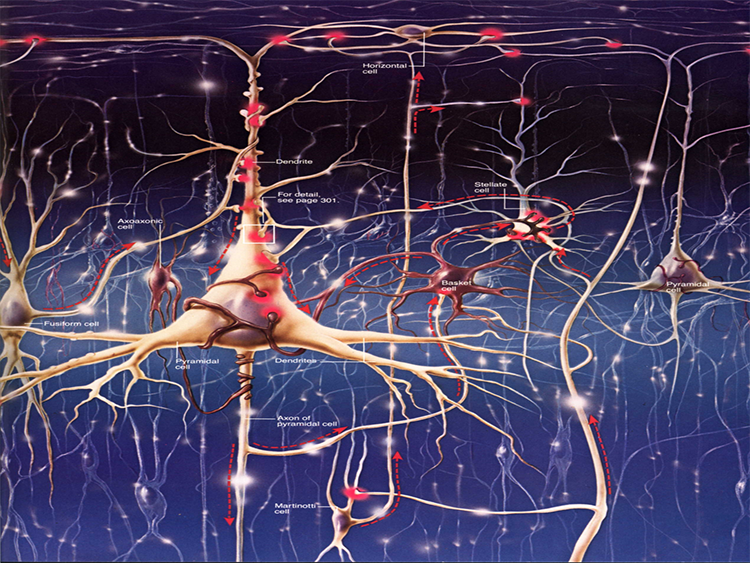
The scalp EEG results from the summation of EPSPs and IPSPs in thousands of cortical columns containing large pyramidal cells perpendicular to the cortical surface. The columns are synchronously polarized (made more negative) and depolarized (made less negative) due to the input of oscillatory or transient evoked activity.
Standardized EEG Assessments
Neurofeedback is a process of identifying and training brain activity that produces the scalp electrical activity recorded by the EEG, so the practitioner must identify common EEG patterns, both normal and abnormal, to facilitate the assessment and training process.
This task has several components and begins with access to an EEG atlas. An EEG atlas is a compilation of “raw” tracings from EEG recordings, showing normal EEG patterns for various age groups and examples of typical abnormal patterns such as epileptiform activity. Examples of normal variants may appear abnormal but have certain characteristics that distinguish them from truly abnormal patterns.
There are several recommended EEG atlases, and they are listed at the end of this section. Many atlases are available, some focusing on childhood EEG, some on adult EEG, and some on the EEG characteristics of specific disorders. Those recommended in this section represent a general overview and include some components of all of the above categories.
The second step is to define normal or typical values for the EEG features so the clinician can use that information to identify training goals and help explain or elucidate causal factors related to client symptoms.
The third step is to proceed with training the client, using the same or similar assessment techniques to monitor progress and to guide any changes that may be necessary as the training progresses. Keep in mind that when making training decisions, they are often informed by identifying excesses or deficiencies at a particular scalp location within a specific EEG frequency band. This doesn’t mean that the assessment and decision-making process ends with this determination. Ongoing recognition of EEG changes, client self-reports, reports from others, and the clinician’s observations of the client will help determine any changes to the training approach.
It is important to note that training decisions must be flexible and fluid. Early in the history of qEEG guided training, some clinicians made the mistake of training to the qEEG, sometimes ignoring client reports of worsening of existing symptoms and/or the appearance of new symptoms. This is a limited and short-sighted approach to training, and the clinician must be willing to shift training if needed to obtain the best outcome. Neurofeedback is as much an art as it is a science. How much weight to give to the qEEG assessment and other assessment tools and the client’s self-report? How often is it appropriate to change training protocols? Must the clinician continue with a prescribed training even if the client reports distress? These are questions that are important to answer. This is one of the reasons that certification programs require that new practitioners participate in a mentoring relationship with an experienced practitioner, to help them provide the most effective, client-centered, dynamic training approach possible.
This brings us to a discussion of the purpose of the EEG assessment. We previously established that neurologists and electroencephalographers use the EEG to identify gross abnormalities, evidence of seizure activity, or the location of lesions in the brain. Those interested in neurofeedback-focused assessments are often interested in more subtle differences in the EEG, leading to useful guidance for training choices.
This is why there may be differences in emphasis between assessments done by a neurologist or research electroencephalographer compared to those done by neurofeedback clinicians. The focus of this section will be to help the neurofeedback clinician utilize the EEG for assessment that guides and informs the training process, including assessment of ongoing progress toward training goals.
Overview of qEEG
Normative Databases
EEG normative databases quantify EEG variables such as amplitude, power, coherence, and phase using EEG samples obtained from healthy normal subjects. These variables are calculated for typical EEG bands or single-hertz bins and all electrode sites and their connections. Values for Brodmann Areas, anatomical structures, regions of interest, and their various connections may also be included.The EEG of an individual client can then be compared to the normative database to see if the client’s EEG deviates to any significant degree from normal. Those EEG variables that deviate from normal may then become targets for neurofeedback training if there is reason to think they are related to the client’s concerns or symptoms.
Normative EEG databases are constructed by selecting data from healthy normal subjects from several age ranges. Subjects are selected for age ranges across the life span because the normal value of EEG variables change during maturation. For example, the amplitude of the eyes-closed delta range decreases as a child ages. Subjects are also selected to exclude conditions that might affect the EEG, such as a diagnosed mental health condition, addiction, or neurological disorder.
Databases usually include samples from eyes-open and eyes-closed conditions because the normal EEG differs under such conditions. Some databases may also include data acquired during cognitive tasks, for example, related to attention, memory, or problem-solving.
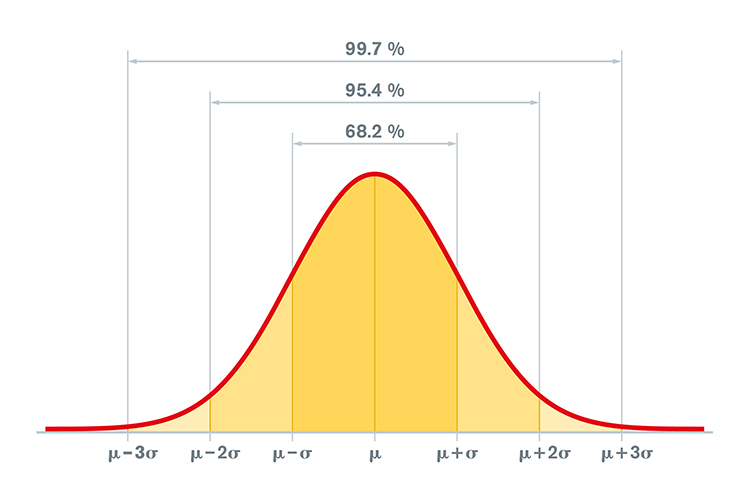
The values of the EEG variables for the database’s subjects are usually transformed into z-scores that are distributed in a bell-like Gaussian curve. In this “normal curve,” most values cluster around the average. Values that are greater than or less than average occur less frequently the more they diverge from the average value. Z-scores are calculated by subtracting an individual’s raw score from the average score of the healthy normal sample and then dividing the remainder by the standard deviation of the sample.
The standard deviation is simply a measure of how variable the data are. A z-score is expressed in standard deviation units of how far a score deviates from the average. When the raw data are transformed into z-scores, a z-score of 0 is the average. Below-average raw scores have a negative z-score, while above-average raw scores have a positive z-score. Approximately 68% of a group’s raw scores will have z-scores between +1 and -1 standard deviations from 0 (i.e., within + 1 SD). About 95% of the group will have z-scores between +2 and -2 standard deviations. Graphic © Peter Hermes Furian/iStockphoto.com.
When a practitioner collects EEG data from an individual client and analyzes their data with a normative database, they calculate the client’s average value of the variable of interest from artifact-free data. For example, after removing artifacts, the practitioner calculates the client’s average number of microvolts in the alpha range with eyes closed.
The number for the client is then compared to the average number of microvolts that the database’s healthy normal subjects of a similar age have with their eyes closed. That difference is divided by the standard deviation of eyes-closed microvolts of alpha for similar-aged normal subjects to produce a z-score.
If the resulting z-score for the client is between +2 and -2, the practitioner may consider the client’s eyes- closed alpha activity to be within the normal range. If the client’s z-score is less than -2, it is significantly deficient or lower than normal. If the client’s z-score is more than +2, it is excessive or significantly greater than normal. The range of z-scores that encompasses “normal” is somewhat arbitrary. For instance, some practitioners use a + 1.5 SD range, and others use a + 2 SD range.
Several companies offer normative databases for clinical and research applications. These include Applied NeuroScience, Human Brain Institute, and qEEG Pro, among others.
Naming the EEG Components
Historically, the naming of the EEG frequencies followed a somewhat chaotic path (Schomer & Lopes da Silva, 2017), beginning with Berger’s (1929) designations of alpha, as the “first” rhythm and beta as everything faster than alpha.
Jasper and Andrews (1938) used the term gamma rhythm for frequencies above 30 or 35 Hz. The term gamma has become somewhat fluid in its definition, depending on the person defining it. Crick (1994) defined gamma as a frequency of 40 Hz (generally defined as 38-42 Hz or 36-44 Hz with a center frequency of 40 Hz), as did Sheer and colleagues (1992). Others, such as Davidson and colleagues (2004), used the frequency band 25-42 Hz to represent gamma, and Swingle also used the term to denote faster beta activity above 25 Hz. Amzica and Lopes da Silva (2017) also used the term gamma to define a frequency of 30-50 Hz. St. Louis and Frey (2016) stated that frequencies from 25-70 Hz are called low gamma, while those above 70 Hz represent high gamma. These sometimes conflicting definitions can lead to confusion as to the precise meaning of this term
Walter (1936) chose the term delta to represent all the frequencies below the alpha band and later added the term theta to identify the portion in the 4-7.5 Hz range. Currently, these terms remain fairly well defined, with delta representing 1-4 Hz and theta representing 4-7 or 4-8 Hz.
Following the naming of these five frequency bands, many other electroencephalographers have sought to name EEG patterns, sometimes choosing names for patterns that already have descriptive designations, such as the pattern known as positive occipital sharp transients of sleep with the acronym POSTS also being called rho waves and posterior slow rhythms of 3-4 Hz being called the pi rhythm. These two terms and several others have fallen out of favor. Schomer and Lopes da Silva (2017) suggest that the use of Greek words to identify EEG activity should be confined to the classic bands of alpha, beta, gamma, delta, and theta. As we will see in our alpha and beta frequencies discussions, the mu rhythm is one exception to this restriction.
A lack of precision in terminology makes numerical designations a better choice for defining EEG observations. This ensures that one’s meaning is clear when speaking of an EEG frequency band. Adding the location and the client’s state (eyes open, eyes closed, asleep, etc.) provides the necessary detail for communicating one’s findings to others. Therefore, describing eyes-closed 8-12 Hz activity, with a dominant frequency of 10 Hz and amplitude between 20-30 uV, in specific occipital and parietal sensor locations, is quite useful for assessment purposes instead of a general finding of “posterior alpha.”
Before proceeding further with the discussions of individual frequencies, it is important to note that brain activity in general and more specifically the scalp EEG patterns that reflect that brain activity are organized and regulated by multiple cortical, sub-cortical, and general network mechanisms. One of the most important mechanisms is cross-frequency synchronization (CFS), which describes waves of one type of frequency, such as beta or gamma, occurring synchronously with the wave patterns of slower frequencies such as delta, theta or alpha. These are often described as nested rhythms. Siebenhuhner and colleagues (2021) propose that cross-frequency synchronization “integrates processing among synchronized neuronal networks from theta to gamma frequencies to link sensory and attentional functions.”
When considering the activity of individual EEG frequencies, it is important to recognize how they interact, how they are interdependent, and how they reflect the activity of the broader neuronal networks in the brain, central nervous system, and the organism as a whole. The EEG does not cause behavior. Instead, it reflects behaviors that have already happened or are currently occurring and are the result of a highly complex dance of interactions.
8-12 Hz EEG – the Alpha Rhythm
Due to alpha being the first EEG pattern to be identified historically, we will begin by defining the alpha rhythm. The International Federation of Societies for Electro-encephalography and Clinical Neurophysiology (IFSECN) (1974) offered the following definition:
Rhythm at 8 to 13 Hz occurring during wakefulness over the posterior regions of the head, generally with higher voltage over the occipital areas. Amplitude is variable but is mostly below 50 μV in adults. Best seen with eyes closed and under conditions of physical relaxation and relative mental inactivity. Blocked or attenuated by attention, especially visual and mental effort.
Such a rhythm must meet all the above criteria to qualify for the designation, eliminating patterns such as mu rhythm in the Rolandic areas, which often has the same frequency but different morphology (characteristic wave shape and pattern) and behavioral correlations.
Source of 8-12 Hz Alpha Activity
Activity in the 8-12 Hz frequency range appears associated with reduced sensory and cognitive activity. Why is this so? What mechanism is responsible for this rhythmic activity?
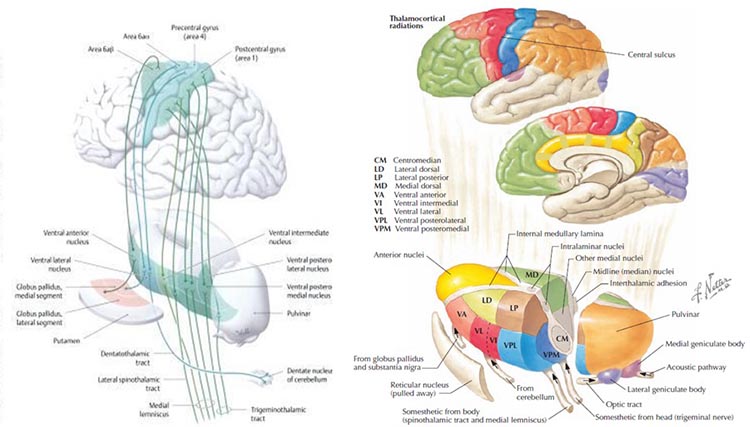
One of the main communication pathways between the external world, the senses that perceive and transmit this information, and the cortical neurons that receive it, is the thalamic-cortical relay system, often designated the TCR system.
The thalamus receives incoming sensory input (a paired structure in the brain's center – see the image below). The individual nuclei of the thalamus transmit that sensory information to appropriate areas of the cortex. The occipital and parietal areas of the cortex are the primary visual processing areas, just as the temporal areas process most of the auditory information. In contrast, central Rolandic areas process tactile and other signals from the skin and muscles. Of course, current findings indicate that brain activity is associated with coordination within and between cortical networks and influences from subcortical structures and local and TCR influences. Still, the TCR system is a primary pathway for determining which areas of the cortex receive each type of sensory input. Thalamic-cortical relay system graphic © Elsevier Inc. - Netterimages.com.
When the eyes close, the neurons responsible for processing incoming visual information no longer have “ work” to do, and so they respond to another signal coming through the TCR system. This is a rhythmic signal mediated by a membrane of inhibitory GABAergic neurons that surround most of the thalamus and provide inhibitory regulation of the signals traveling to the cortex. This is called the reticular nucleus of the thalamus (TRN) or nucleus reticularis of the thalamus (NRT). The function of this system is much too complex for this section, but a good treatment is available in Crabtree (2018), and an examination of the role of the TCR and TRN systems in consciousness is found in Min (2010).
The rhythmic signal from the TCR and NRT interaction produces a 10-Hz (8-12 Hz range) input to the visual processing neurons when visual sensory input is withdrawn (eyes closing), resulting in those neurons firing synchronously in that frequency in response to this input. The voltage of a specific EEG frequency, measured at the scalp surface, is directly proportional to the number of cortical neurons firing synchronously in that frequency (Nunez & Srinivasan, 2006). Therefore, when the eyes are closed, and the signal from the TCR system changes from sensory input to a rhythmic 10-Hz input, visual neurons respond to that input and the voltage of alpha, and most specifically in adults, 10-Hz activity increases in voltage. The image below shows a spectral display of an eyes-closed EEG.
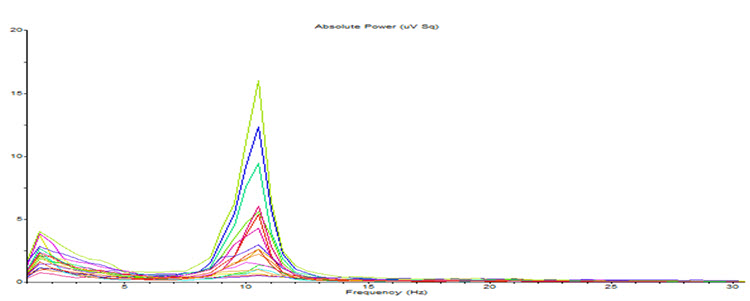
Note: eyes-closed EEG in longitudinal bipolar montage represented as a spectral display. The x-axis shows frequency from 0-30 Hz, and the y-axis shows absolute power (uV Sq). Note the peak at about 10 Hz. Voltage is higher in the P3-O1 derivation than in the P4-O2 derivation, revealing a small asymmetry. The highest power is in the T4-T6 derivation.
Identifying alpha activity in parietal and/or occipital areas of the scalp is usually quite easy, particularly in the eyes-closed condition. The image below shows an eyes-closed alpha pattern from a 15 -year-old male.
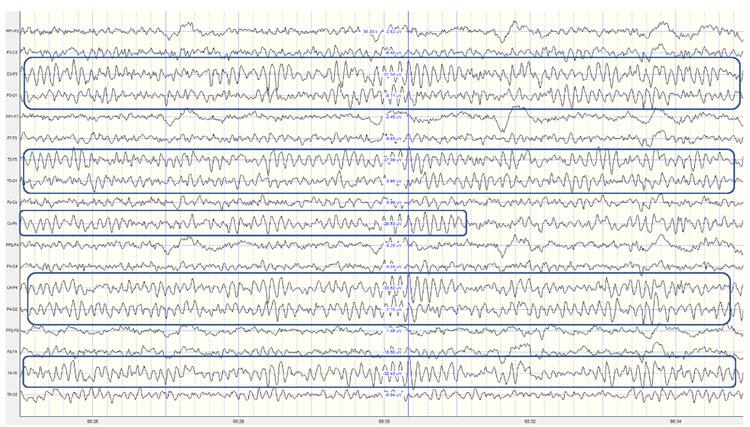
Note: eyes-closed EEG filtered to 1-45 Hz in the longitudinal bipolar montage with a 50-uV scale. Boxes indicate the most prominent 8-12 Hz activity. This montage represents a series of adjacent electrode comparisons or derivations, as each signal tracing is derived from each pair of electrode comparisons.
This longitudinal bipolar montage is displayed below.
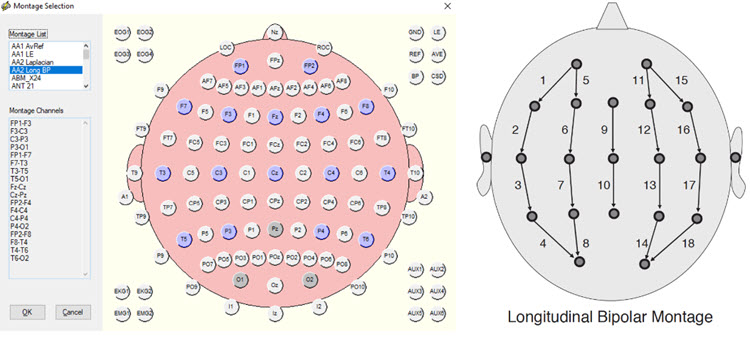
Note: longitudinal bipolar montage (commonly known as the “double banana” montage). Note the arrows in the right-hand image indicating the sequence of pair-wise comparisons. Various sequences have been used.
Observe that the rhythmic activity is well-defined and has the typical bursting or spindling pattern of the alpha rhythm, resulting from the input of the TCR and NRT systems. Spindling consists of a series of distinct oscillations of a particular frequency that begin with relatively low amplitude, increase in amplitude, and then decrease in amplitude, giving the appearance of a spindle such as one used in spinning, with fiber wound around it. Graphic © New Africa/Shutterstock.com.

The waves are quite sinusoidal (waving up and down in a smooth rhythm similar to a sine curve) and continue throughout the recording with minimal disruption. The voltage indicator shows that the maximum voltage at the moment of the line placement was from about 20 to 30 uV at the peak of the waveform in this montage.
One can determine the wave's frequency by either counting the number of peaks in a one-second segment or counting the number of times the wave crossed the zero line and dividing by 2 (zero crossings/2). Either method gives a 8-9 Hz value when multiple one-second epochs are counted. This is somewhat slow for a 15-year-old, although the voltage appears to be within normal limits. When compared to a normative database, we can see that indeed, it is a slow peak alpha when compared to other 15-year-old males as indicated by the chart below from the NeuroGuide database:
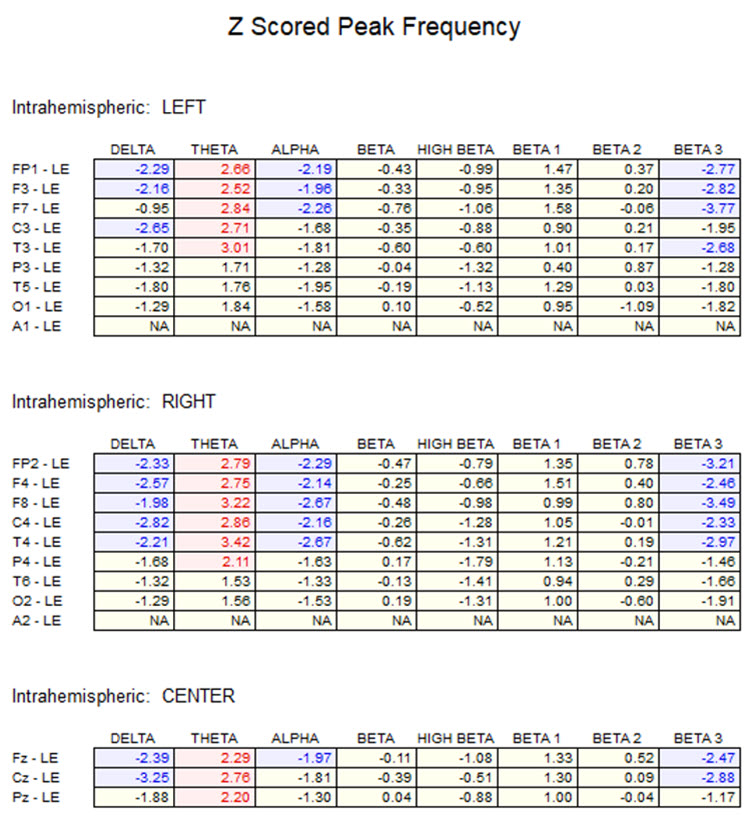
Note: the alpha peak frequency z-scores are at least 1 SD below expected values (> -1.0 SD) in all locations and many areas show deviations exceeding the significance cutoff of -1.96 SD (blue highlight). The database also plots the theta peak frequency as fast (red highlight) due to slow alpha in the 8-Hz frequency bin or segment. There are likely other slow components of the dominant rhythm that contribute to this incorrect plot of the frequency information. Ideally, the peak frequency of the EEG should be calculated within a broad range from approximately 6 Hz to about 14-16 Hz to avoid this type of error. This is another problem with the somewhat arbitrary designation of frequency bands using set values.
The same data processed by the iSynchBrain database show similar findings for O1 and O2 below:
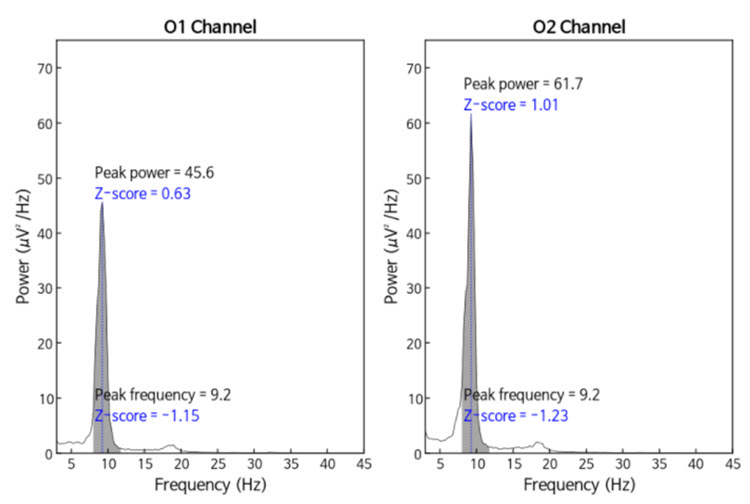
Note: peak frequency power and frequency comparisons at O1 and O2 showing slow peak frequency at 9.2 Hz bilaterally, resulting in z-scores of -1.15 and -1.23, respectively, with voltage (power) on the left at 0.63 SD and on the right at 1.01 SD, showing slightly elevated values. This database provides single-Hz calculations for both frequency and amplitude rather than using an arbitrary band to define alpha.
The alpha peak frequency is age-dependent, though not called ” alpha” until the frequency reaches 8 Hz. In early infancy, it is designated as the posterior basic rhythm or posterior dominant rhythm (PDR). It appears around the age of 4 months with a frequency of about 4 cycles per second (c/s) or Hz (Schomer & Lopes da Silva, 2017). The PDR increases (speeds up) during maturation and is approximately 6 c/s at 1 year and up to 8 c/s at around 3 years of age. This is when it can be called the alpha rhythm.
The frequency reaches 10 Hz by approximately 10 years of age, and that (10.2 ± 0.9/sec) is the peak frequency of adulthood (Petersén & Eeg-Olofsson, 1971). From the previous example, we see why a peak frequency between 8-9 Hz is slow for a 15-year-old.
Meaning and Importance of the Alpha Peak Frequency
The speed or frequency of the alpha peak frequency is often mentioned, even in a neurologist’s report. For the neurofeedback practitioner, it is helpful to understand the factors associated with different alpha frequencies. The alpha peak frequency is a measure of the frequency of the rhythmic pattern of the posterior rhythm (generally called alpha). This has traditionally been an important measure. Although it has recently been somewhat de-emphasized in some EEG circles, it remains an interesting measure. It has a great deal of research supporting it as a useful metric for assessment.
A slow peak alpha frequency has been associated with some forms of cognitive decline and memory impairment (Stam, 2017) as well as mild traumatic brain injury (mTBI; Jabbari et al., 1985; Williams, 1941). A fast peak alpha frequency has been associated with improved scores on timed IQ tests. It has also been associated with enhanced memory and cognitive performance in various age groups (Grandy et al., 2013). A faster peak alpha frequency is associated with advanced reading skills in precocious children (Suldo et al., 2001).
The peak alpha frequency changes through the lifespan. Therefore, age-normed values for the peak alpha frequency are important for assessment purposes. The normal adult peak alpha frequency is 9.5-10.5 Hz. Jabbari et al., 1985 Scholarly literature states that the peak alpha frequency is not abnormal until it is below 8 Hz (Schomer & Lopes da Silva, 2017). However, it is commonly thought to be potentially meaningful when the frequency is below 9 Hz for an adult.
Stam (2017) stated that slowing the alpha peak frequency by more than 1 Hz (9 Hz for an adult) is generally a sign of pathology. A slow alpha frequency can be associated with fatigue, cognitive decline, and memory impairment. Slowing of the background alpha rhythm is also a sign of generalized cerebral dysfunction (Nayak & Anilkumar, 2021). Rathee and colleagues (2020) related the speed of the peak alpha frequency to reading comprehension. They found that a slower peak alpha frequency is associated with poor comprehension.
Negative correlations of a peak alpha frequency faster than 10.5 Hz, possibly associated with an overly activated central nervous system, may include sleep initiation problems, anxiety, intrusive thoughts, and difficulty with self-soothing and self-calming skills.
Meaning and Importance of the Eyes-Closed Alpha Response Voltage
In addition to the peak frequency within the 8-12 or 8-13 Hz alpha band, the amplitude of the activity can also be meaningful. The alpha voltage will be partially affected by the montage that is used. For example, keeping in mind the discussion of differential amplifiers in the Instrumentation and Electronics section, the closer two electrodes are to each other, the more the rhythmic, synchronous patterns will be attenuated. Common-mode rejection (CMR) is most sensitive to frequency synchronization, so waves that are the same frequency and also synchronous (e.g., 10 Hz waves, waving up and down at the same time at the two sensor locations + and – [also commonly called “active” and “ reference”]) will be rejected. Comparing the O1 and O2 occipital electrodes to each other will result in a lower apparent voltage of alpha if the two waveforms are synchronous, which is quite likely.
Conversely, comparing either O1 or O2 to an ear reference or possibly to a forehead reference would result in almost no rejection of alpha activity. Therefore, the rhythmic patterns are unlikely to be similar at these distant locations and will be retained. When viewing standard voltage information in an atlas or a research paper or textbook, try to identify the montage used when those standards were developed.
Simonova et al. (1967) found amplitudes between 20 and 60 μV in 66% of their subjects, while values below 20 μV were found in 28% and above 60 μV in only 6%. Schomer and Lopes da Silva (2017) suggest that values between 10 and 60 μV are typical. However, other sources such as the John Hopkins Atlas of EEG (2011) and Libenson’s Practical Approach to Electroencephalography (2009) cite 20 μV as the minimum voltage for adults. These differences may seem insignificant but can represent the difference between a low voltage fast EEG finding and a typical assessment.
Rhythmic alpha activity represents the synchronization of the EEG. It represents part of the excitation/inhibition cycle. When either large or small groups of neurons perform tasks, this results in the desynchronization of the EEG during work, as each group of neurons performs its function somewhat locally and somewhat independently. This is followed by a resting or inhibitory phase that results in the synchronization of the EEG and hence an increase in alpha amplitude as many neurons fire synchronously. This is clearly seen in the shift from active visual processing when the eyes are open to a synchronous pattern of oscillatory activity when neurons do not have incoming visual input to process and can rest. This measure of alpha voltage change from eyes open to eyes closed, known as the alpha response, and the decrease of alpha with eyes opening, called alpha blocking, helps identify if the work/rest cycle is occurring correctly.
Someone with an eyes-closed posterior dominant rhythm voltage below 20 μV suggests to the neurofeedback assessor that the person does not easily shift to a state of decreased arousal/alertness necessary for alpha amplitude to increase. The disconnection from the outside world upon eyes closing should result in decreased sensory processing of vision and other senses and decreased cognitive activity, leading to an increase in 8-12 Hz amplitude or power.
Typically, alpha activity voltage should increase as more neurons fire synchronously in this frequency. When this increase is less than 50% above the resting baseline eyes-open alpha voltage, it usually indicates some difficulty turning off the mind, meaning that neurons remain activated and working and thus prevented from entering a resting state. The lack of a typical alpha increase may be associated with heightened states of alertness and vigilance, meaning that these clients maintain their external perceptive focus and/or cognitive activity, even when the eyes are closed, likely meaning that the ability to achieve global synchronous activity is being inhibited. Resulting behavioral consequences can include fatigue, as the neurons are constantly engaged and are not allowed to rest. This pattern may be associated with a history of trauma and/or a history of hypervigilance for various reasons.
Example of a typical alpha response upon eyes closing:
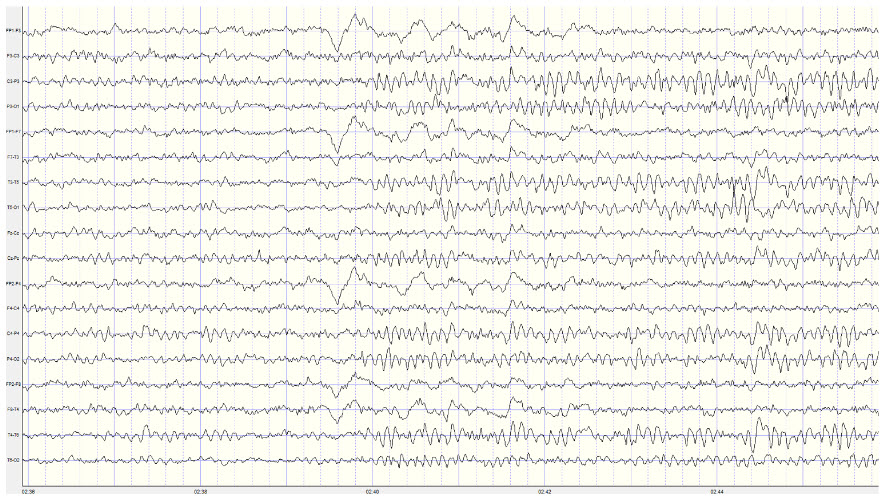
Note: this is a 19-channel recording of the transition from eye-open to eyes-closed conditions. This is a longitudinal bipolar montage, and the scale is 50 μV. Note the eyes closing at minute 2:40 (indicated by the eye movement) and the immediate response of the alpha rhythm appearing.
Meaning and Importance of the Alpha-Blocking Response
Conversely, the continued presence of alpha once the eyes are open suggests a lack of appropriate alpha blocking. This appears to result from a lack of inhibition of synchronous generator mechanisms. Hartoyo and colleagues (2020) showed a simple mechanism: excitatory input to inhibitory cortical neurons. This differs from the excitatory cortical neurons that typically reduce synchronous cortical firing in favor of local responses to incoming stimuli when the eyes are opened.
Note that activation of inhibitory mechanisms results in increased inhibition, even though the function is initially excitatory. Conversely, activation of excitatory mechanisms results in greater activation. This can seem confusing, and it may help to focus on the result, whether excitatory or inhibitory, rather than the initial behavior.
There should be a dynamic balance between excitation and inhibition in the human neocortex (Dehghani et al., 2016). When this balance is disrupted, we see the behavioral effects noted here. Alpha blocking represents the re-activation of visual processing neurons when visual input returns. Typically, as these neurons are no longer in a common or general resting state but are involved in task-oriented behaviors that are more localized, synchronous activity should decrease (be inhibited). Therefore, the overall voltage will decrease because of less synchronization. This does not imply that more neurons are firing when alpha amplitude is higher; it means less synchronization and hence lower voltage.
Imagine an auditorium full of people initially clapping synchronously (eyes-closed alpha rhythm). The noise is loud because everyone is clapping at the same moment (higher amplitude) and completely quiet in between claps, and the frequency of the claps reflects that synchrony. Then, imagine everyone clapping independently, possibly in synchrony with immediate neighbors but not with the audience as a whole. This is like the eyes-open condition and, though there will always be some noise, because someone is always clapping, resulting in a faster frequency of clapping. At no one moment will it be as loud as when the entire audience clapped synchronously. So, the overall voltage is lower even though the frequency of clapping is faster. Thus, alpha amplitude decreases when the eyes are opened, and this should occur quickly, in 1-2 seconds and certainly in less than 10-15 seconds. Any delay in alpha blocking suggests difficulty returning to the task. Desynchronization of the EEG occurs in posterior areas as visual processing begins when the eyes are opened.
The most common reasons for the lack of appropriate alpha blocking (meaning alpha activity persists after eyes are opened) are:
1. Fatigue, including sleep deprivation
2. Long-term meditation practice, particularly mantra meditation
3. Marijuana use and abuse, generally long-term, chronic
4. Cerebral dysfunction due to disease, injury, or possibly chemical exposure
Below is an example of correct alpha blocking following the eyes opening:
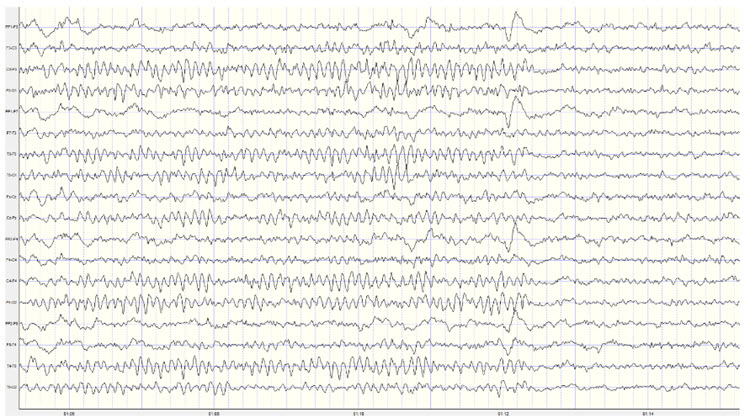
Note: this is a 19-channel recording of the transition from eyes-closed to eyes-open conditions. This is a longitudinal bipolar montage, and the scale is 50 μV. Note the eyes opening at minute 1:12 (indicated by the eye movement) and the immediate blocking of the alpha rhythm.
Clearly, the response of 8-12 Hz EEG activity can be quite revealing and provides the clinician with helpful information about the client. However, it is important to note that other factors can affect the EEG recording. We have already noted the effects of artifacts on the EEG generally. Additionally, the client’s state of mind, level of anxiety, comfort with the application of sensors to the scalp, level of trust of the practitioner conducting the recording, amount of sleep, the use of caffeine and other stimulants and common medications can all affect the results of the recording.
Once an assessment is made of excess or deficient alpha activity, lack of an alpha response or persistent alpha following the eyes opening, or a slow or fast peak alpha frequency, the clinician can proceed with training to address these findings. There are multiple approaches to training the 8-12 Hz frequency band, including training specific segments of that band to achieve training goals. For example, if a lack of an alpha response to eyes closing is associated with anxiety and possibly insomnia, training for an increase in the 8-10 Hz portion of the posterior alpha rhythm in the eyes-closed condition may be an effective intervention. If the peak alpha frequency is slow, training for increases in the 10-12 Hz portion may help speed up this frequency. If there is persistent alpha in the eyes-open condition, inhibiting or downtraining 8-12 Hz generally may be helpful.
Of course, with any intervention, other causal factors must be addressed as well. Persistent alpha and/or frontal alpha can signify fatigue secondary to a sleep disorder such as sleep apnea. Therefore, a referral to a physician for a sleep study may be helpful. Over-arousal patterns that correspond to a lack of alpha response can be associated with a history of emotional, psychological, physical, or sexual trauma. These issues may need to be addressed by the neurofeedback clinician or by referring the client to an appropriate therapist.
13 or 14 to 25 or 30 Hz EEG – the Beta Rhythm
The following description is from Kane et al. (2017):
Beta band: Frequency band of 14–30 Hz inclusive. Greek letter: β.
Beta rhythm or activity: Any EEG rhythm between 14 and 30 Hz (wave duration 33–72 ms). Most characteristically recorded over the fronto-central regions of the head during wakefulness. The amplitude of the fronto-central beta rhythm varies but is mostly below 30 µV. Blocking or attenuation of the beta rhythm by contralateral movement or tactile stimulation is especially obvious in electrocorticograms. Other beta rhythms are most prominent in other locations or are diffuse and may be drug-induced (for example, alcohol, barbiturates, benzodiazepines, and intravenous anesthetic agents).
Beta activity appears to result from multiple factors associated with active patterns organized by slower rhythms. Bursts of beta occur in frontal-central, frontal-temporal, frontal-parietal, and central-parietal areas and are associated with different tasks based upon the structures underlying these areas. Because all functions are distributed to multiple sites, it is difficult to precisely define which part of the CNS is responsible for each function. The observations in this section are based on both animal and human studies, the latter primarily in lesion studies and during neurosurgery.
Beta is also associated with the negative shift of the DC gradient (Speckmann et al., 2017). The DC gradient, only measurable by a DC-coupled EEG amplifier, measures the overall electrical gradient of cortical areas under the recording sensors. This gradient shows a slow oscillation of less than 1 second and is usually in the 0.1-0.2 Hz range. A shift of the gradient from its current state, becoming more electrically positive or negative, occurs every 5-10 seconds and sometimes quite a bit less often. Buzsaki (2011) states that there is a progression of frequencies whose bandwidths overlap and interact with each other, from frequencies that take 15-40 seconds to complete each cycle up to those that oscillate at 200-600 cycles per second. Gunkelman (2005) called beta and gamma activity “emergent properties of bound networks.” This means that, as slower frequencies of EEG synchronize across networks, beta and gamma emerge in bursts of activity in coordination with that synchrony.
Beta activity is associated with "work" and reflects the ongoing excitatory/inhibitory cycles occurring on multiple time scales. While the work/rest cycle mentioned in relation to the alpha response and alpha blocking is one type of excitatory/inhibitory cycle on a broader scale, beta activity reflects actions that occur millisecond by millisecond and appear to be more locally generated.
Beta frequencies have a relatively low voltage compared to slower frequencies. As noted in the above definition, amplitudes are generally less than 30 μV and frequently less than 20 μV. Remember our example of the audience clapping. In the example below, “clapping” occurs more often but with less power due to less synchronous activity.
Below is an example of eyes-open beta activity:
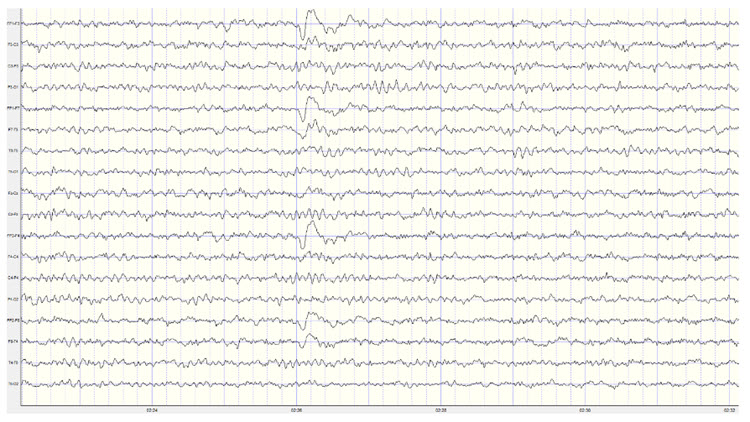
Note: this is a 19-channel EEG recording in the eyes-open condition. This is a longitudinal bipolar montage, and the scale is 50 μV. Observe the alpha activity continuing at a much lower voltage in parietal and occipital derivations, compared to the previous eyes-closed examples, resulting from the attenuation of alpha with the opening of the eyes. There is beta activity in the 20-30 Hz range at less than 10 μV, seen mostly in frontal, central, parietal, and temporal derivations (comparisons between adjacent sensors) and somewhat slower 15-20 Hz patterns.
Kropotov (2016) describes the existence of several beta rhythms with different frequencies, various locations, and distinct functions. From this information, he states that there is likely no single neuronal mechanism for generating localized beta activity. This fits with the understanding that beta activity is associated with local tasks and therefore is mediated more by local mechanisms, all with overall coordination from network systems and other rhythmic activity.
When beta activity is typical for the client based on age, state (eyes-open, eyes-closed, under task, etc.), and location, it suggests that those areas are functioning as expected. If beta activity is deficient, that may mean that the site is under-functioning or under-activated for some reason. Reasons may include damage of some sort, metabolic deficits, fatigue, or other factors. An area that is consistently over-functioning may, in time because of overuse and fatigue, end up with a lower level of functioning and hence less beta activity.
Higher-than-typical beta amplitude at a given location may mean that the area is over-functioning or overly activated. Whether the beta amplitude is higher or lower than average, the clinician will want to understand the underlying functional neuroanatomy of the area to aid in the assessment process. For example, suppose the right posterior temporal/parietal junction that roughly underlies the area between T4 and T6 (TP8 in the 10-10 system) shows excess beta amplitude. In that case, it may be associated with heightened sensitivity to or attention to non-verbal communication such as tone of voice, facial expression, and body language associated with angry outbursts or that may signal danger. If this area is under-activated, it may represent a self-protective “disconnection” from these same signals (Gunkelman, 2021).
Individuals who typically show excess beta activity at sleep onset and during sleep stages have a higher incidence of insomnia (Perlis et al., 2001). Meier and colleagues (2014) found a correlation between excess beta power in frontal, central, and temporal areas with delinquent behavior in adult men with concurrent ADHD symptomatology. In Rowan’s Primer of EEG (2nd ed.), Marcuse and colleagues (2016) identify excess interhemispheric beta asymmetry as an important diagnostic tool. The side with reduced relative beta power points to the pathological hemisphere. They identify brain abscesses, stroke, tumor, vascular malformations, and cortical dysplasia as associated with a focal decrease or enhancement of beta activity.
A reference to a normative database can be useful when assessing beta activity, particularly if a traumatic brain injury is suspected. Comparison with other EEG frequencies is also important, as an excess or lack of beta activity is often accompanied by differences from expected values for other frequencies.
Rhythms with Similar Frequencies But Different Characteristics – Not Beta
There are a few special cases where activity in the supposed beta frequency range may represent other EEG patterns. One important example is a rhythmic pattern seen over the sensory-motor cortex, generally at C3 and C4, known as the mu rhythm. This rhythm has a frequency of between 10-15 Hz and, therefore, may be mistaken for alpha or beta activity. Kane and colleagues (2017) describe the mu rhythm as follows:
Mu rhythm: Rhythm at 7–11 Hz, composed of arch-shaped waves occurring over the central or centro-parietal regions of the scalp during wakefulness. Amplitude varies but is mostly below 50 mV. Blocked or attenuated most clearly by contralateral movement, the thought of movement, readiness to move or tactile stimulation.
Greek letter: μ. Synonyms: rhythm rolandique en arceau, comb rhythm (use of terms discouraged).
The mu rhythm can occur with a frequency over 13 Hz and can have spectral peaks in alpha (8-13 Hz) and beta (14-25 Hz) frequency bands (Jenson, 2020) and hence appear in either the alpha or beta frequency bands in quantitative EEG analysis. Mu can also show an additional characteristic, known as a harmonic effect in the EEG recording (Cheyne, 2013; Jones et al., 2009). It usually manifests as a peak in a spectrograph at twice the frequency of the mu rhythm. So, a 10-Hz mu rhythm would often have a 20-Hz pattern associated with it, whereas a 13-Hz mu rhythm may show a 26-Hz harmonic.
Magnetoencephalography (MEG) studies by Jones and colleagues (2009) show that what they call mu-alpha (mu rhythm in the alpha frequency range) and mu-beta (mu rhythm in the beta frequency range) co-occur at a rate greater than chance, suggesting similar mechanisms associated with their presence in the recording. They also show that this activity is not an artifact of the recording process but that it is more difficult to differentiate the faster beta component when using EEG instead of MEG. MEG studies have demonstrated that beta frequency activity originates in precentral locations and alpha frequency activity in post-central locations (Hari et al., 1997).
Below are two examples of mu rhythm activity as a tracing with a 19 channel recording and as a topographic representation of the same data.
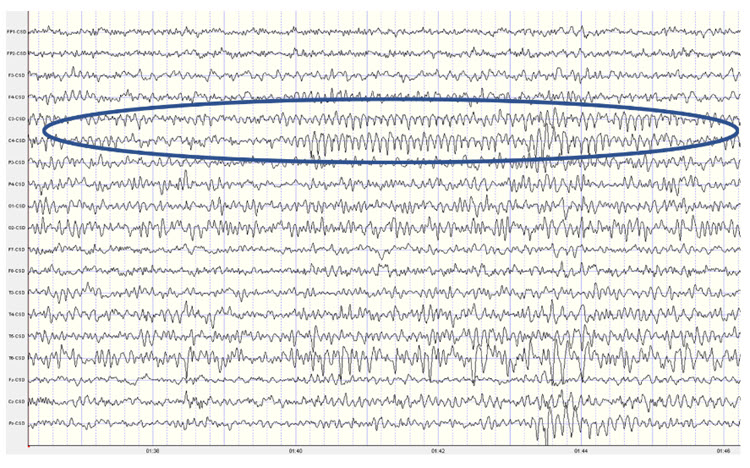
Note: this example shows a mu rhythm with the typical negative-going wave (up in this example) appearing somewhat rounded and the sharp positive-going wave (down) at C3 and C4. This is a Laplacian montage with a scale of 300 uA (microamperes). Counting the frequency yields a mu rhythm of 10-11 Hz.
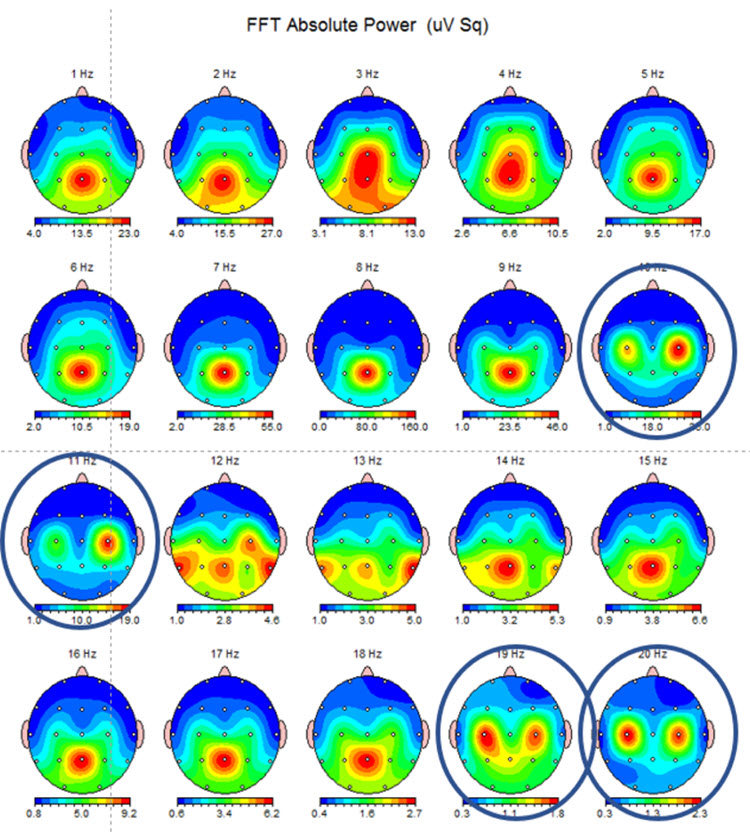
Note: this example shows the same EEG data in a topographic display showing the 10-11 Hz mu activity at C3 and C4 and the double harmonic at 19-20 Hz in the same locations. This is often referred to as the “owl eye” display because the presence of mu rhythm at C3 and C4 produces a display that looks like the face of an owl.
The appearance of the mu rhythm over the sensory-motor cortex has been associated with pathology (Gastaut & Bert, 1954; Gastaut et al., 1959), such as psychosomatic symptoms in individuals identified as neurotic. Chatrian and colleagues (1959) demonstrated that suppression of mu activity is associated with one’s movements or when observing someone else moving. In the early 1950s, mu was thought to be quite rare, and various studies identified the prevalence of between 2.9-14% (Schnell & Klass, 1966). In an early version of the textbook Electroencephalography by Niedermayer and Lopes da Silva, the incidence of mu was estimated at approximately 18%. More recent studies using computerized spectral analysis and coherence calculations (Kuhlman, 1978; Schoppenhorst et al., 1980) show the presence of mu in 60% of their study participants.
Mu activity has been correlated with motor cortex functions, as noted above. It appears to be similar to the alpha activity described in posterior areas in response to eyes-open and eyes-closed conditions. Similarly, mu activity seems to be present when the motor cortex is idle and blocked or attenuated when the motor cortex becomes active. Interestingly, mu is blocked with the physical movement of the person being recorded, when that person observes movement, and when the person even visualizes movement. However, unlike alpha, mu activity does not block with eye-opening.
Mu activity has been associated with what has been called the mirror neuron system (MNS), which is a network of locations that are associated with what might be termed learning by observation, mimicry, or imitation. Bernier and colleagues (2007) identify mu as reflecting an underlying execution/observation matching system and studied the relationship of abnormalities in response patterns of the mu rhythm in individuals with autism spectrum disorder (ASD). They show decreased attenuation of mu rhythm when adult individuals with ASD observed physical movement in others compared to age- and IQ-matched typical adults. Montirosso and colleagues (2019) show different patterns of mu desynchronization between pre-term and full-term infants at 14 months of age during an action observation/execution task, with full-term infants showing more broadly distributed areas of mu rhythm desynchronization (blocking of mu due to cortical excitation) compared to the pre-term infants.
Okada and colleagues (1992) identified patients with mu rhythm activity that increased with drowsiness, photic stimulation, and hyperventilation as being more likely to experience intractable epilepsy or to suffer from organic brain disorders compared to another group with more well-controlled epilepsy and psychiatric disorders where the mu rhythm showed more typical behavior, i.e., not blocking with eye-opening but blocking appropriately with spontaneous movement or with sensorimotor stimulation.
Finally, Siyang and colleagues (2016) have correlated mu with blood oxygen level-dependent (BOLD) signals. They have shown that mu activity's higher power (amplitude) is negatively correlated with the BOLD signal over the sensorimotor network, the attention control network, and the mirror neuron system. This means there is a decrease in blood oxygen utilization when the mu rhythm is higher in amplitude, confirming its nature as a resting state indicator for these systems and networks. Additionally, higher mu amplitude was positively correlated with the BOLD signal in areas of the salience network such as the anterior cingulate and anterior insula. So, it appears that some systems are at rest when mu is active, and some are more active when this is true. This, again, speaks to the complexity of EEG activity and the global, system-wide relationships associated with all EEG frequencies.
Thus, mu rhythm has become an area of increased study and interest, and training mu activity over the sensory-motor cortex has become a fairly common neurofeedback intervention. For example, a psychiatric clinic in Nashville, TN has seen improvement in multiple symptoms when Rolandic mu is trained using z-score neurofeedback (Tim Caldwell, 2020).
The visual identification of mu rhythm activity can be helpful, and task-oriented assessments that show the response to movement or observations of movement should be included in the EEG assessment of potential clients.
There is a similar pattern of activity in the sensory-motor cortex in the 12-15 Hz range that some identify as mu rhythm but which is also labeled as the sensory-motor rhythm (SMR). This is a pattern noted by Sterman (1967) and reported in multiple publications regarding his work with cats (Sterman & Enger, 2006) and subsequently in his work with human participants (Sterman, 1996). Sterman initially trained cats to increase this EEG frequency activity and found that it appeared to prevent seizure activity in the presence of toxic substances. He then taught human participants with intractable epilepsy to produce increased amplitude of SMR with excellent results, and this work was replicated in multiple publications. An excellent review of the history and development of this area of EEG research and training is available in Enger and Sterman (2006).
Neurofeedback training of SMR eventually progressed to working with ADHD clients (Lubar & Shouse, 1976) and others. This pattern is associated with decreased motor excitability and is similar to the sleep spindles seen in stage 2 sleep. Clients learning to increase the voltage of this 12-15 Hz pattern show a marked decrease in hyperactive behaviors. Training in this frequency band is commonly used for clients with ADHD hyperactive or combined subtypes. It is included within the broad, generic beta frequency band. Still, it shows specific characteristics that suggest it doesn’t have the same behavioral or local generation characteristics that more typical beta activity seems to have.
Below is an example of SMR activity in an eyes-open recording.
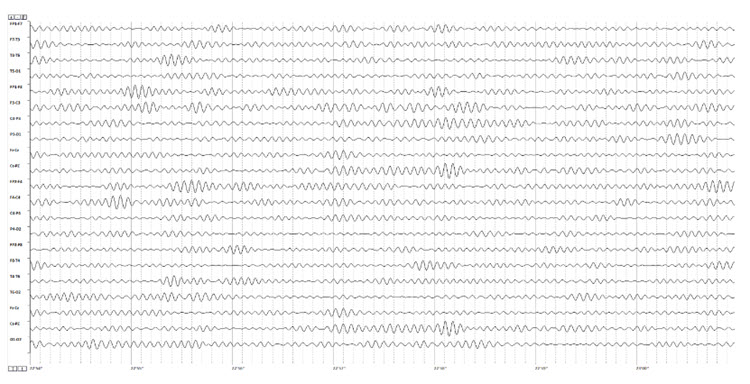
Note: this is an image of a filtered EEG recording – isolating only the 12-15 Hz frequency band. The montage is longitudinal bipolar, and the scale is 10 uV due to the low voltage of this signal. Observe that activity in this band is present in all electrode locations but does show more of a persistent bursting or spindling pattern in derivations involving central electrodes.
The 12-15 Hz (SMR as defined by Sterman) EEG over the sensory-motor cortex was one of the first EEG patterns to become the focus of neurofeedback training. As noted, many published studies demonstrated the efficacy of training this frequency band in central electrodes for conditions as diverse as seizure disorders and attention deficit disorder. Training this frequency continues to be a commonly used intervention in the neurofeedback field. The controversy regarding whether SMR activity is the same as Rolandic mu or a distinct pattern with unique characteristics is difficult to resolve. When viewing SMR activity in individuals with prominent mu rhythm, the bursting patterns do not appear synchronous, suggesting a separate mechanism of action. Still, these are simply the author's observations and have not been verified by rigorous studies.
The presence of more precisely identified frequency patterns in the EEG with more clearly defined behavioral correlates (e.g., the beta frequency band) is a good reason to be wary of broadly defined EEG frequency bands with a lack of specificity by location or behavior. We will also see this occur in the other frequencies that are covered in this discussion.
The assessment of beta activity includes location specificity and differences between locations, so training choices also target these findings. When training is focused on correcting atypical results, attention must be paid to compensatory behaviors reflected in the EEG. This will be discussed in more detail toward the end of this section.
0.5-3.5 Hz or 1-4 Hz or 0.1-4 Hz – The Delta Frequency Band
Continuing with our somewhat historically-defined EEG frequency discussion, we move on to the delta frequency band of 1-4 Hz or sometimes defined as 0.5-3.5 Hz and even as 0.1-4 Hz. Kane (2017) describes delta as follows:
A frequency band of 0.1–<4 Hz. Greek letter: δ. Comment: for practical purposes, the lower frequency limit is 0.5 Hz, as DC potential differences are not monitored in conventional EEGs (due to the use of AC amplifiers rather than DC-coupled amplifiers for most EEG recordings – author’s note).
This somewhat limited description is partially due to the difficulty of defining cellular activities associated with the EEG from 0.1-4 Hz. Amzica and Lopes da Silva (2017) discuss the delta frequency band in some depth and conclude that the activity in this frequency range represents more than one phenomenon and that frequency-band definitions do not reflect the underlying mechanisms of the various sub-components of this activity.
Amzica and Lopes da Silva state that activities associated with delta frequencies reflect two different EEG phenomena, waves and oscillations. They describe oscillation as a repeated variation of a parameter such as current or voltage between two values with the possible additional characteristic of a regular pattern to that variation. They define a wave as a single variation of a parameter between two extreme values and state that oscillations appear to be made up of waves. Current thinking identifies at least two cellular sources of delta, the thalamus and the cortex.
Thalamic delta oscillations result from two inward currents of thalamocortical cells (Soltesz et al., 1991). These currents appear to be associated with low-frequency membrane potential oscillations in thalamocortical cells that continue even in vitro after removal from the organism. This was identified in studies in rat and cat subjects (Leresche et al., 1991) and verified in human studies during in vivo monitoring (Crunelli et al., 2018). There are intrinsic mechanisms within these cells that result in regular electrical discharges. Crunelli and colleagues show that these oscillations have a rhythm-regulation function as expected and a plasticity function that can shape ongoing oscillations during inattention and NREM sleep, reconfiguring thalamic-cortical networks to facilitate information processing during attentive wakefulness.
Cortical delta oscillations continue even when those neurons are disconnected from thalamic input. Again, this suggests an intrinsic mechanism within cortical neurons that allow these very slow rhythms to occur even without communication from other sources. Grey Walter (1936) identified delta activity in the scalp EEG overlying areas of cerebral tumors. More recently, localized delta activity has been associated with areas of traumatic brain injury (Buchanan et al., 2021). This is one area where quantitative analysis of the EEG can be extremely helpful when evaluating individuals post-stroke or post-mTBI. Areas disconnected from local and/or global networks generally show increased delta rhythm activity and do not function as expected. Neurofeedback training can often help resolve these disconnections or facilitate a reorientation of function to a different set of neurons.
Activity in the delta frequency range appears to represent multiple brain functions. As noted earlier in the discussion of cross-frequency synchronization, delta appears to play a significant role as an underlying pacemaker for organizing and coordinating everything from local activation that results in beta frequencies to global network functions that integrate local information processing results from multiple sensory areas, to interpretive, problem-solving, and decision-making activities, to command-and-control outcomes.
These broadly distributed functions that must be coordinated in both time and space require an underlying physiological mechanism to facilitate such coordination. Crick (1994) suggested the 40 Hz (gamma) rhythm as the binding mechanism, and others have also recommended this. Gunkelman (2005) suggested the glial system as the physiological mechanism and the slow cortical gradient and delta frequencies as the EEG components that reflect the activity of this system, with gamma as an emergent property that only appears when the slow cortical gradient/delta system is bound or synchronized.
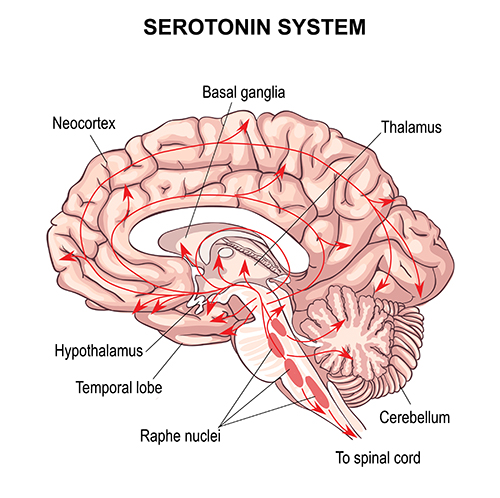
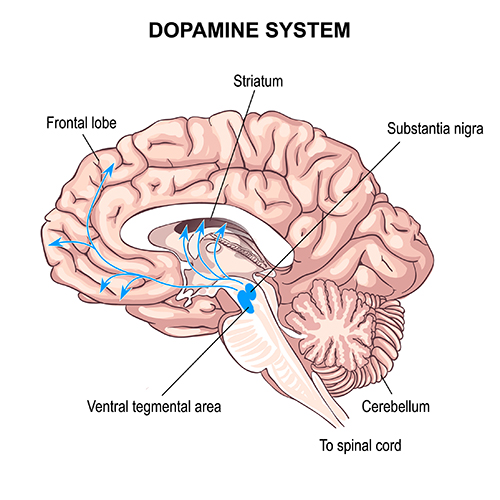
This latter concept is supported by more recent findings regarding glial cells. Recent neurophysiological understanding of brain function has been focused on chemical synapses, through which neurons communicate, and other systems and subcortical mechanisms influence cortical activity. This sub-cortical influence is seen in the ascending pathways from brainstem areas that project to the thalamus and cortex. Two examples, shown below, are serotonergic and dopaminergic pathways.
Serotonergic pathways
Dopaminergic pathways
The glia are linked in a gap junction-based network, with glial cells connecting through direct electrical coupling via proteins known as connexons that form gap junctions. Alvarez-Maubecin and colleagues (2000) have demonstrated the same type of connection between glia and neurons. Gap junctions are capable of nearly instantaneous communication instead of the relatively slow communication in the neurochemical synaptic transmission system. Thus, large and broadly-distributed networks of gap junction-linked glia can communicate and organize neuronal activity by generating slow oscillations mediated by calcium and potassium concentrations and by glia-to-neuron gap junction communication. This is in marked contrast to the notion that the slow oscillation results from neuronal activation and reflects the effect of postsynaptic potentials on the local field potential. Amzica and Lopes da Silva (2017) clearly show the neuronal activation that follows glial influences. The synchronization between the two reflects this global coordination mechanism.
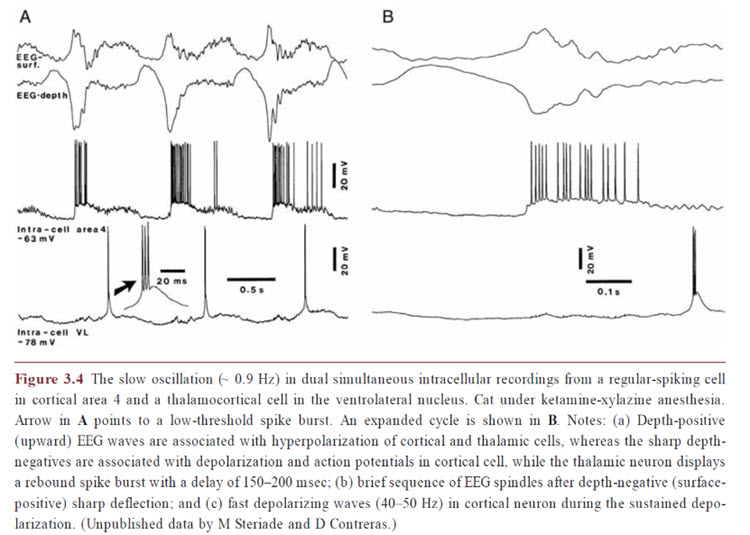
Delta activity appears to be a component of this slow oscillation, particularly at the slower delta frequencies below 2 Hz. The intrinsic cellular oscillations noted earlier occur at these less-than-2-Hz frequencies and likely should be viewed independently from the faster components of the delta band. These oscillations likely result from the glial involvement discussed above, which appears to continue even when neurons are not receiving sensory input. See the image below from Niedermeyer’s (2017) Electroencephalography of unpublished data by Steriade and Contreras showing the correlation between neuronal activity and the slow cortical oscillation.
Again, we see that more precise and specific discrimination of EEG frequency activity and the physiological and behavioral correlations associated with those patterns can be helpful when analyzing EEG recordings. This is also true of subsequent training approaches.
The slow cortical gradient or slow cortical potential (SCP) has been studied concerning the incidence of cortical activation. There is a clear correlation between greater cortical negativity (an electrically negative shift) and increased neuronal firing. When the cortex shifts to a more electro-negative state, this lowers the firing threshold for cortical neurons and increases cortical excitation. The opposite is true, and cortical positivity is associated with reduced neuronal firing.
Kotchoubey and colleagues (2002), in collaboration with Neils Birbaumer of the University of Tubingen in Germany, showed reduced seizure frequency in participants with refractory epilepsy when trained to create an electro-positive SCP shift, with the goal of reducing cortical excitability and hence reducing the frequency and intensity of seizure activity. In a 10-year follow-up of study participants (Strehl et al., 2014), experimental group participants continued to show reduced seizure frequency compared to the initial pre-study baseline. Those participants were also able to demonstrate the same control of the SCP gradient in three follow-up training sessions. This sustained improvement occurred without any intervening “booster” training sessions. Other studies of the usefulness of SCP gradient training for migraines and ADHD have also been conducted.
Training the cortical gradient has also been a component of the various ultra-slow and infra-slow neurofeedback training approaches discussed in the Selecting Training Protocols section. Though they don’t generally reference the cortical gradient directly in discussions of their approaches, the mechanism of action is likely associated with the glial system communication and organization properties.
Clients identified with excess localized delta activity and traumatic brain injury, stroke, or other focal abnormalities have improved from neurofeedback training as noted earlier. Local training to inhibit or downtrain the delta activity at the site or sites of abnormally high voltage is one approach. Still, more recently, a global approach to neurofeedback training via sLORETA and swLORETA neurofeedback seeks to correct network-wide dysregulation, including errors of connectivity which, as mentioned earlier, may underly the local finding of excess delta activity. This highlights the need for a comprehensive assessment, including an EEG assessment that shows connectivity metrics that can help recognize global disruptions that may need to be addressed.
Regarding the delta frequency band assessment, some artifacts mimic both delta and slow cortical gradient patterns. These include lateral eye movements that may represent normal nystagmus (slow drift or slow phasic shift of the eyes laterally when the eyes are open) or similar shifts when the eyes are closed. Saccades or quick side-to-side eye movements are faster and less likely to be mistaken for delta waves during visual inspection or artifact removal.
Another artifact that influences the slower component of the delta band, as well as the slow cortical gradient, is skin electrodermal activity, variously called the skin conductance response (SCR), electrodermal response (EDR) or galvanic skin response (GSR). This skin response activity shows a fairly slow oscillation at rest, reflecting tonic sympathetic nervous system (SNS) activity, and also shows more phasic responses to stress or challenge. The slow, tonic change in the skin's conductive properties changes the electrical conductivity under the EEG recording electrode on the scalp. It can cause an apparent (although false) slow oscillation in the cortical gradient measure and the delta frequency band recording under certain conditions.
Another issue, particularly when recording the SCP, ultra-low or infra-slow frequency ranges, is the presence of electrode drift, which is a function of metals used in EEG electrodes that can lead to unstable signals due to erratic ion exchange. This is most commonly seen in tin electrodes, which are unfortunately quite frequently used, particularly in EEG electrode caps. They are solid electrodes rather than coated with a conductive substance that can wear off. Other materials such as solid silver and gold-plated silver have varying degrees of drift as the electrode/conductive gel/skin surface interfaces come into equilibrium. The best electrode material to reduce electrode artifact is the sintered silver/ silver chloride (Ag/AgCl) electrode, a mixture of silver and chloride compressed into a solid pellet. The second best is an Ag/AgCl-coated electrode. However, the coating can wear over time. For a full analysis of electrode materials and drift, see the excellent study by Tallgren and colleagues (2004).
Finally, older EEG amplifiers, particularly those using long EEG leads from the scalp to the amplifier, show a phenomenon called cable sway when EEG leads move through the electromagnetic field of the recording environment. Leads can sway due to the movement of the client, movement by the EEG technician, or even from air currents. These cable sway movements can result in fairly high amplitude and slow oscillations that can be mistaken for SCP and delta activity.
4-8 Hz - Theta Frequency Band
The last of our basic frequency bands is known as theta. The very brief description from Kane (2017) is below:
Theta band: Frequency band from 4-8 Hz. Greek letter: Ɵ
Theta rhythm: Rhythm with a frequency of 4-8 Hz.
Theta wave: Wave with a duration of 1/4 to over 1/8 s (125–250 ms)
EEG activity in the theta frequency band is quite specific to the location where it is recorded. 4-8 Hz activity in temporal areas has different functional and behavioral correlates from the same frequency activity in frontal midline or posterior areas. This, again, reaffirms that location and behavior are essential components when analyzing scalp EEG.
Below is an example of filtered (4-8 Hz) theta activity:
Note: this is an eyes-closed recording in the longitudinal bipolar montage with a 20-uV scale. The EEG is displayed using a 4-8 Hz filter to isolate that frequency band from the full band EEG. Observe the slightly greater amplitude and rhythmicity in temporal derivations.
Below is 1-45 Hz display of the same EEG recording at the same time location:
Note: this is an eyes-closed recording in the longitudinal bipolar montage with a 50-uV scale. The EEG is displayed using a 1-45 Hz filter to show the relatively full EEG band.
Amzica and Lopes da Silva (2017) cite various studies regarding the theta rhythm they identify as 4-7 Hz. As discussed earlier, they note that normal theta activity should not be confused with pathologic theta, which represents a slowing of the alpha frequency band into the theta range. They suggest that this slowing of alpha may result from reducing cerebral blood flow or metabolic encephalopathies. Metabolic encephalopathies can result from chemical imbalance due to various causal factors from kidney or liver dysfunction, diabetes, or a variety of other health issues (MyHealthAlberta.com).
Arnolds et al. (1980) found significant differences between behavioral conditions when viewing hippocampal theta recorded with depth electrodes. Writing resulted in faster frequency and greater rhythmicity but lower amplitude than sitting or walking. In contrast, a word association task resulted in faster frequency, greater rhythmicity, and increased amplitude in the period of silence immediately following the question but before the answer was given.
Ekstrom and colleagues (2005) studied hippocampal and neocortical theta activity during a virtual driving task that involved location finding. They found that both areas increased theta during all tasks associated with the driving simulation. A significant correlation between all areas showed increased coordination between multiple areas while accomplishing the tasks. They concluded that both cortical and hippocampal theta oscillations and coordination between these areas are associated with attention and sensorimotor integration.
Historically, the study of attention disorders has focused on excess frontal theta activity in individuals with inattentive ADHD. The ratio of theta (4-8 Hz) activity to beta (13-21 Hz) activity, or the theta/beta ratio (T/B ratio), was developed to make the analysis of this metric easier. It was initially calculated using a single channel, vertex location at Cz (Monastra et al., 1999). Other studies compared multiple locations and found that the Cz location was accurate and represented the location of the largest deviation of the ratio between previously diagnosed ADHD clients and typical controls (Lubar, 1991). Identifying an elevated T/B ratio (meaning more than typical theta compared to the amount of beta) compared to typical controls appeared to be an accurate way to assess attention disorders.
In a large, blinded, multi-center validation of the theta/beta ratio in comparison with rating scales for the assessment of ADHD, the researchers calculated the sensitivity and specificity of measures. They found that the T/B ratio achieved superior results than commonly used rating scales (Snyder et al., 2008). With a sample size of 159 individuals, the EEG assessment showed a sensitivity of 87% and a specificity of 94%, for an overall accuracy of 89% compared to the next closest measure, the Connors’ Rating Scale – Teacher version (CRS – Teacher), with a sensitivity of 67% and specificity of 41%, for an overall accuracy of 58%.
The sensitivity of an assessment measure determines how accurate it is at identifying individuals known to have a particular condition. Specificity is a measure of how accurately the measure correctly eliminates individuals without the condition from identification as having the condition.
For example, the Connors’ Rating Scale – Parent (CRS – Parent) shows a sensitivity of 78%, meaning that it identifies 78% of clients known to have ADHD. However, it does this at the expense of identifying 86% of typical controls as also having ADHD (specificity of 14%). This could mean that many typical children could be diagnosed with ADHD and possibly treated with medication if the CRS-Parent was the only tool used for such an assessment – something that is quite often the case. Even if the teacher version of the rating scale were used, it would still result in 59% of typical children being misdiagnosed (specificity of 41%).
When using the T/B ratio, only 6% of typical children were incorrectly identified. This suggests that, at a minimum, the use of a simple, single-channel EEG assessment should be included as a component of a comprehensive approach to identify ADHD children before prescribing medication.
Interestingly, Van Son and colleagues (2019) expanded the associations that could be identified with the T/B ratio. They determined that a higher T/B ratio was negatively correlated with prefrontal executive control, including response inhibition and negative affect control. This suggests that excess theta in relation to beta activity could lead to greater impulsivity and to a lack of control of negative behaviors. They also found an association with higher T/B ratios and reward-motivated decision making, possibly selecting immediate gratification at the expense of long-term benefit. Finally, they correlated the T/B ratio with increased mind wandering, decreased executive network functions, and increased default mode network (DMN) activity.
As with all the previous EEG frequencies and assessment measures, the correct amount of a particular frequency activity is important. For example, someone who lacks appropriate default mode functioning may experience a lack of the type of resting-state activity that appears to have a therapeutic effect. The DMN has also been called the resting state network (RSN) due to its functions that differ from task-oriented behaviors. It is thought to be important for a variety of reasons. Therefore, a person with a lower-than-typical T/B ratio may benefit from increased theta voltage and some training in activating the DMN. In contrast, an individual diagnosed with ADHD may benefit from training to reduce or inhibit excess theta voltage.
Temporal lobe theta likely reflects the hippocampal theta identified using depth electrodes. It appears to be associated with route finding and navigation, both hippocampal functions. Differential, interhemispheric (bipolar montage) training of temporal lobe areas in the theta frequency range has been a component of certain approaches to neurofeedback for some time (Othmer & Othmer, 2007). This approach is used for various conditions, including migraine, tinnitus, PMS, and many others. Frequencies are adjusted to facilitate the optimal response and may range from the alpha frequencies, through the theta frequencies, down to the infra-low frequencies below 1 Hz.
Again, the analysis of theta activity is often aided by using a normative database. Otherwise, determining of whether an amount is too high or too low in amplitude is difficult to discern. Fortunately for T/B ratio assessment, Monastra (2001) has provided a table of values with three age ranges, 6-11 yrs, 12-15 yrs, 16-20 yrs. The table of mean T/B power ratios is below:
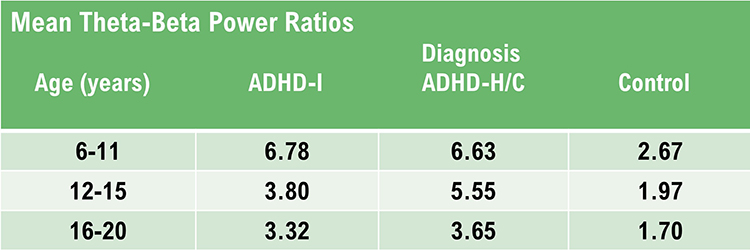
Note: ADHD-I = attention deficit-hyperactivity disorder, inattentive type; ADHD-H/C = attention deficit-hyperactivity disorder, hyperactive-combined type.
Assessment of theta activity more generally, beyond the T/B ratio in the central midline is more challenging. As noted in Van Son (2019), assessment under task may be essential to determine these results more accurately.
Final Notes
The recognition of standard EEG frequencies, the analysis of such activity, and using this information for training and re-assessment purposes is an important part of neurofeedback practice. This section has attempted to provide an overview of this area to aid the new practitioner and the experienced clinician alike in furthering their understanding of this complex area of study.
One of the greatest benefits of neuroscience in general and electroencephalography and neurofeedback specifically is that they encourage lifelong learning. If this section appeared overwhelming, with few hard and fast rules or concrete facts to hold on to, it is important to keep in mind that one can do useful and effective neurofeedback even without an in-depth understanding of this area. Understanding of EEG comes gradually through regular exposure to educational materials, lectures and workshops, work with an experienced mentor, and regular interaction with clients and their EEG recordings.
There is no substitute for viewing large numbers of EEG recordings. As mentioned in the beginning, this can start with an EEG atlas and then progress to examining your own recordings, whether a single channel, a couple of channels, or multiple channels.
Patience with one’s process is an integral part of any learning experience. Think of the time it takes to learn any worthwhile skill, from swimming to learning a musical instrument to learning a new computer or phone operating system. Managing one’s expectations is crucial.
A Few Final Notes on the Evaluation of the EEG – Compensatory Behaviors
As mentioned earlier, when evaluating the EEG, keep in mind that when an area of the brain is not functioning optimally, other areas may increase their activity to compensate. This is also true of emotional and psychological factors. The client may have developed coping strategies that result in unexpected EEG findings. These include excess right hemisphere activation in beta frequencies, excess alpha in left frontal areas or lack of alpha response in posterior areas. When training to resolve these differences in EEG activity, keep in mind that differences from typical do not necessarily mean pathological. Taking away a compensatory skill that serves the client well and not causing additional disorder is not a desirable approach. It is one of the reasons for being careful when training to a quantitative EEG with database comparison. It is important to match symptoms with findings so that truly troublesome issues can be addressed.One example of the effective application of this principle is Thatcher’s surface and LORETA neurofeedback program within the Neuroguide program. This system utilizes a symptom checklist matching function that correlates qEEG findings with the client's presenting symptoms and then only targets those areas with the greatest correlation to both symptoms and findings. Other systems will likely be developed to accomplish this same goal, and an experienced mentor can also provide similar guidance.
Training Approach
The next step is to develop a training approach to help the client resolve symptoms and issues that appear to be related to the findings.
There are almost as many approaches to this training process as there are neurofeedback practitioners. This may seem confusing and gives some ammunition to those claiming that any reported neurofeedback benefits are only due to the placebo effect.
However, this is not the case. Neurofeedback is the process of providing information to the client in a form that they can understand and that represents changes in the EEG. When this information (feedback) is presented to the client with some instruction about the desired direction of change and with feedback that rewards the desired behavior, the client learns a new skill through this interactive process. This is similar to any learning endeavor. Learning a musical instrument requires a great deal of repetitive practice and feedback from a teacher and the instrument itself. As long as the student can tell when a note is 'wrong' for that particular piece of music, then that instant feedback can be used to play the note correctly the next time it is attempted.
With neurofeedback, the feedback is so quick as to be nearly instantaneous. This allows the brain to associate the internal behavior with the feedback and, through trial and error, allows the brain to learn to produce the correct behavior – play the correct note reliably.

The brain practices this particular skill repeatedly until it becomes a habit. However, making a particular pattern of brain activity appear on the scalp surface under a specific electrode or multiple electrodes requires the entire brain and nervous system, including all of the command and control mechanisms, networks, and sub-cortical structures, to become engaged in the process.
Once the skill has been learned, of course, the level of global engagement decreases dramatically. This is true of any skill-learning process. Large energy expenditures are required at first, and then reduced expenditure occurs once the neuronal mechanisms that embody the skill have become 'hardwired' into the nervous system. The goal is for the skill to become automatic so that the client does not have to expend a great deal of energy to accomplish the task.
Therefore, as with physical exercise, almost any exercise (neurofeedback training) program can be beneficial as long as it is done carefully, is mindful of the client's capacity, and closely listens to the effects. So, multiple approaches to the same client issue can have beneficial effects if the above cautions are followed.
Nevertheless, the individual practitioner must still decide where to put the sensors, which frequencies to train, whether to train frequencies up (increase voltage) or down (decrease voltage), whether to train one area or function at a time or to train multiple areas, functions, frequencies and EEG metrics like coherence and phase in addition to amplitude.
In the early days of quantitative EEG, a "brain map" would be consulted, areas of excess activity would be trained to decrease that activity, and/or areas of deficient activity would be trained to increase that activity. This was often done with a single sensor location and continued until the area showed normal activity.

Other early practitioners based their training on the client's symptoms and the understood location in the brain where that symptom was likely to reside. This approach relied greatly on published neurology literature, electroencephalography research, and other sources to determine the best place to train. Again, this was often done with a single sensor location but tended to be more concerned with the client's self-report about the resolution of symptoms.
Both of these approaches continue to this day, though often with various variations as individual practitioners use their experience to fine-tune their approaches for specific client complaints.
In addition to these brief descriptions of often very complex approaches, approaches have appeared based upon real-time comparisons to normative databases to guide the training. This is commonly known as z-score training, as it trains EEG variables toward more typical values (standard deviations) when measured against the normative database. A standard deviation (SD) of zero would represent the mean but usually, anything up to 1.5 to 2 SD is within the typical range. Variables, like the amplitude of a single EEG frequency band or multiple variables at multiple locations, that exceed 2 SD can be trained to move toward z = 0. This can be done using up to 19 sensor locations simultaneously, training hundreds of variables and/or multiple systems associated with brain networks.
It is common for adherents of these approaches to be skeptical of the other approaches, sometimes quite vocally. However, each approach appears to result in desirable change for the client. It would be difficult to do a comparison study to determine which, if any is superior since training is always focused on the individual client. Even though there are commonalities among clients, it would be difficult to eliminate all the variables, so only the type of neurofeedback training could be studied.
This next section will cover each of these approaches in some detail to provide the reader with information to make some initial decisions.
Client Training
When we discuss client training, the field of neurofeedback becomes somewhat confusing, as noted above. In this section, we will attempt to give new practitioners methods to determine a relatively easy starting point for their developing practice.
One of the simplest training approaches, used in many published studies, involves training to increase the amount or voltage of 12-15 Hz at the central midline location known as Cz. The individual EEG frequencies are discussed elsewhere in qEEG Tutor, and each frequency band's behavioral characteristics are also described.
Briefly, the 12-15 Hz frequency band was identified by Barry Sterman and colleagues, who labeled it the sensory motor rhythm or SMR. Increasing this frequency band requires a decrease in task-oriented activity in the sensory and motor cortices, leading to a physical and cognitive calming effect in most people. Individuals with a lack of activation in these areas will often experience increased alertness and physical calming, which is why it has been frequently used for those with a diagnosis of attention deficit hyperactivity disorder (ADHD), or in the older designation, attention deficit disorder or ADD.
Several published studies showed improvements in academic scores, improved behavioral self-regulation, and reduced distractibility (see the meta-analysis by Arns et al., 2009). This same training protocol was initially identified as helpful for seizure disorders, and multiple published studies in reputable journals replicated those findings.
The Case for Multiple Approaches
We recommend that clinicians learn multiple approaches so they have the knowledge and skill to help clients with varying presenting concerns. This includes the use of additional biofeedback interventions such as heart rate variability training, EMG, peripheral skin temperature and galvanic skin response biofeedback, and more. Three strategies are the symptom-matching approach, and two qEEG-guided strategies, the clinical database and normative approaches.Symptom-Matching Approach
The symptom-matching approach requires the student to learn a great deal of information before starting to train clients. Some experienced clinicians have tried to address this issue by providing a "decision tree" approach to recommended training. These take the form of workbooks or guides that provide an "if this, then do that" format with a series of steps to be followed if initial interventions do not provide adequate relief.A valiant effort by Susan Othmer (2006) produced such a resource in her Protocol Guide for Neurofeedback Clinicians that is still much sought after but which is now out of print. The 2019 edition focuses more on the infra-low frequency training approach. This approach has many practitioners, mostly trained by Sue and Siegfried Othmer, and has generated much controversy. However, those who use this approach claim a high level of efficacy.
Paul Swingle has written a series of similar books to try to guide new practitioners, such as Biofeedback for the Brain (2010) and Adding Neurotherapy to Your Practice (2015). Consider John N. Demos' (2019) Getting Started with EEG Neurofeedback (2nd ed.) and Richard Soutar and Robert Longo's (2022) Doing Neurofeedback: An Introduction (2nd ed.). Finally, read Michael P. Cohen's (2020) Neurofeedback 101: Rewiring the Brain for ADHD, Anxiety, Depression and Beyond (without medication).
qEEG-Guided Approaches
A more thorough analysis using a quantitative assessment of the EEG allows for the selection of training protocols based on more objective information. Two basic approaches rely on qEEG. One is a clinical database approach, and the other is a normative one.Clinical Database Approach
The clinical database approach is a set of assumptions based on the clinician's knowledge, experience, and training and which have been organized into an easy-to-administer assessment, usually producing correlations with the findings and the client's symptoms. A provider can use this information to identify the most important areas for neurofeedback training. The quantitative components of this analysis produce information such as ratios, measures of differences from one location to another, peak frequency information, maximum voltage, minimum voltage, and other metrics. All help to determine how the client's EEG differs from expected characteristics and what can be done to restore optimal functioning.
Normative Database Approach
The normative database approach is based upon a large sample of typical individuals, grouped into age and sometimes gender cohorts, whose EEG has been recorded and entered into the database using a variety of mathematical algorithms to ensure statistical validity. The client's EEG can then be compared to this "normal" sample, and variations can be identified, leading to locations and frequencies to train. The normative database generally does not provide a report linking client symptoms to the findings, but in the case of the NeuroGuide TM database, a symptom and findings matching system has been developed to guide z-score neurofeedback training.
Clinical and normative databases have been covered in much greater detail in qEEG Tutor's Database Analysis unit, so that this brief overview will suffice for this section.
The quantitative assessment of the EEG takes many different forms. The transition from the visual inspection of the EEG to the quantitative analysis of the EEG has been an interesting journey and has taken many directions. Research into the quantitative analysis of the EEG has proceeded independently of the neurofeedback field in many cases, and the goal of these researchers has been to both identify new methods for such analysis and verify and validate the accuracy and reliability of these methods. This has helped neurofeedback practitioners to feel confident in the methods they use and also find new metrics both for assessment and training. The future of qEEG-guided neurofeedback training is quite bright and exciting.
Interpreting Quantitative Assessment Information
The following segment will cover the interpretation of the quantitative information generated by the various assessment tools. Subjective factors such as expectation biases will always be present in such reviews and analyses. We have learned certain ways of looking at information of all types, from a sunrise to a facial expression to a qEEG topograph. In many cases, these allow us to work more quickly and automatically. We don’t have to reinvent the wheel or learn to tie our shoes repeatedly. Our brains recognize patterns and learn to seek out important information at a glance and to separate meaningful information from noise. However, this also limits our ability to see and interpret new information that we haven’t been exposed to previously. This is why it is important to learn from many teachers and to engage in an ongoing learning process to keep our perceptions fresh and flexible.
We will begin with a fairly simple, six-location assessment collected sequentially using two sensor locations simultaneously. This is known as the NewQ and has been developed and revised over about 10 years by John Anderson. The following graphs represent the results of the assessment in a spreadsheet format.
Note that a disclaimer at the top urges the practitioner to use caution and good clinical judgment when evaluating the results.
General Description: This EEG assessment is completed in one series of sequential measurements. Locations include P3, P4, C3, C4, F3, and F4, measured in two-channel pairs.
Each two-channel pair will have associated behavioral components such as eyes open, eyes closed, mental math, physical movement, and relaxed attention. The data derived from these behavioral periods will be analyzed in the post-recording computation and then pasted into an Excel or LibreOffice Calc template for analysis.
Review results
1. Results are seen in Columns I through M.2. Column I indicates the task or behavior being measured, such as eyes closed following the eyes-open or other task condition.
3. Column J indicates the client’s response.
4. Column K indicates the expected response.
5. Column M provides information regarding typical or unexpected responses.
6. These descriptions are suggestions only and must be verified by comparing them with actual client symptoms. If the client denies the symptoms, they should be tabled until further analysis can be conducted.
7. The descriptions of possible symptoms associated with each finding often list multiple examples. The client may not have all these symptoms and may only have one or two or none. It is best to interpret the results with the client and preface this process by noting this factor.
Visual Inspection after Recording
It can be helpful to visually inspect the recording to look for an artifact or interesting rhythms such as mu. The following graph shows two columns of information – column I shows what is being measured in the EEG, and column M explains why it is measured and what the findings represent. Following this graphic is a full spreadsheet of an actual client’s results. Please note that an artifact removal process occurs before selecting data for analysis.Line by Line Explanation of NewQ Template Fields (Column M)

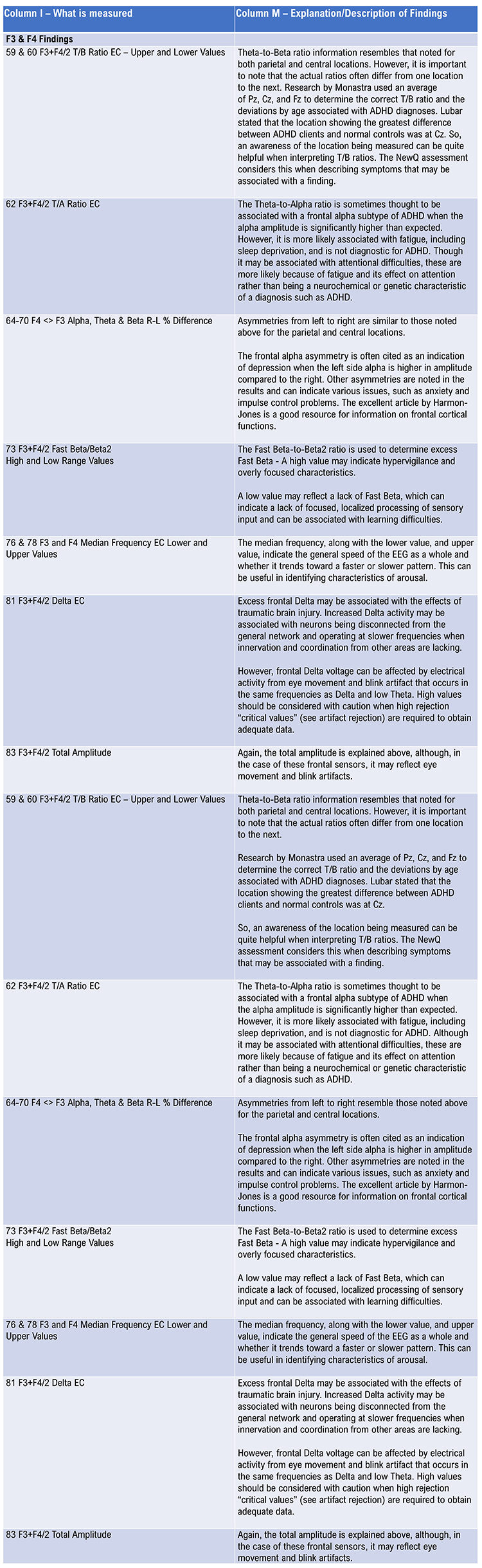
Below is an example of the NewQ results spreadsheet populated with client data showing only columns I-M. Column I represents the EEG characteristic being measured, column J shows the client’s response, column K shows the expected response, and column M describes symptoms and possible causal factors associated with the findings. Note that in column K, when the value IAT Calc* is shown, the expected value depends on many factors that are calculated using an “if, and, then” (IAT) sequence and cannot be represented by a standard value.
This has been a description of one example of a clinical database assessment. Several others, some quite extensive, use more electrode sites and produce graphic displays of the results.
From this analysis and discussions with the client, it appears that her primary issues are worrying, rumination, anxiety, and sleep problems – mostly stemming from the previous issues. She reports getting 4-6 hours of non-restful sleep each night and waking to feel quite tired. She has a history of emotional abuse in her family of origin and states that she has always felt anxious.
Training choices for this client will likely address these issues by helping her learn self-calming skills, beginning with heart rate variability biofeedback training that involves both respiration training and training to find her resonant frequency with combined respiration and heart rate feedback. Please see the in-depth treatment of these topics in HRV Biofeedback Tutor.
Additional biofeedback training could include peripheral temperature and galvanic skin response (GSR) training and EMG training to facilitate reduced muscle tension.
Finally, neurofeedback training can focus on encouraging the transition to sleep through parietal training at 6-9 Hz, commonly known as Alpha-Theta training. High frequency 13-36 Hz and low frequency 2-6 Hz inhibits can be used to help encourage decreased anxious rumination and to prevent negative reactions that sometimes result from the client disconnecting from conscious awareness by "going too deep." Other neurofeedback training protocols could include downtrain-ing left frontal alpha and theta activity and right-sided beta and/or downtraining frontal fast beta. This could also be accomplished using frontal z-score training or, more generally, 19-channel swLORETA neurofeedback focusing on the anxiety network.
Database Analysis Results and Interpretations
Next, we will evaluate the results of an example normative database comparison and interpret the results.As described elsewhere in qEEG Tutor, a typical normative database uses 19-channel recordings (additional reference and ground channels are implied) that allow for various derived values that become a part of the results produced for each client. We begin with an analysis of the visual signal of the 1st recording in the eyes-closed (EC) condition.
The eye movement artifacts shown above are quite common in eyes-closed recordings and do not represent EEG activity. Computers and EEG software cannot distinguish the source of electrical activity. Activity that occurs at the measured frequency is displayed and would produce results that suggest excess amplitude in that frequency at that location. See below two images from the NeuroGuide software showing a JTFA analysis of EEG data at the location of the vertical blue line. The first one shows what a statistical topographic map would look like if eye-movement artifacts were included, and the second one shows the artifact-free EEG.
In the first image, the topographic maps show significant (> 2.5-3 SD) excess theta and delta activity in the pre-frontal and lateral frontal areas that are actually eye movement, so not real EEG.
The three images below each show the same selection. The first image in the linked ears montage (LE) shows excess alpha activity due to reference contamination, while the average reference and Laplacian montages do not. Reference contamination is discussed elsewhere in qEEG Tutor.
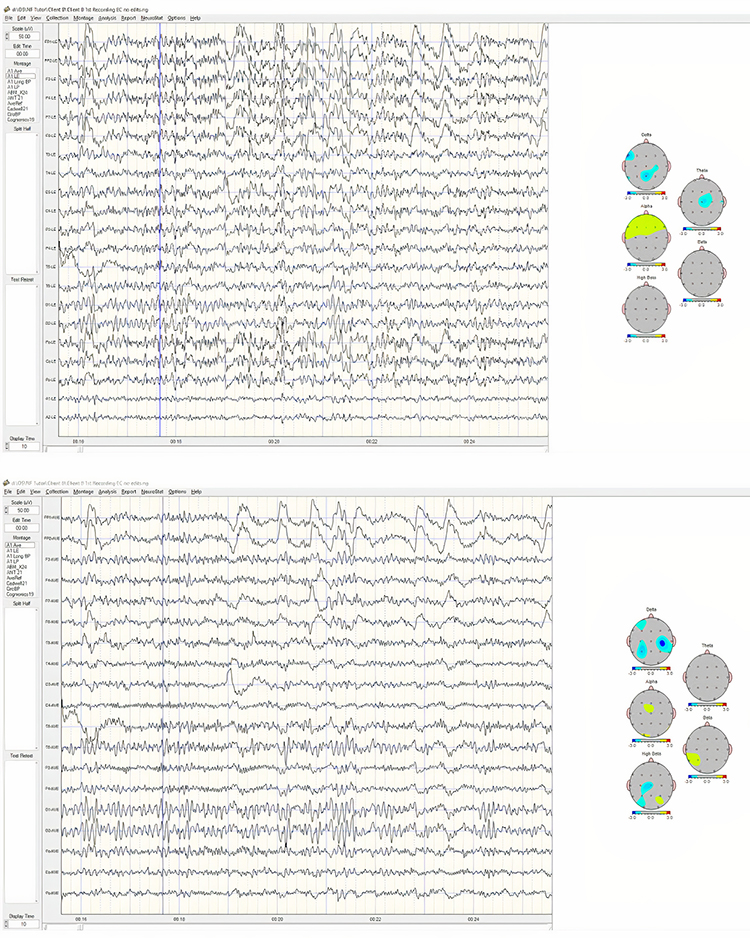
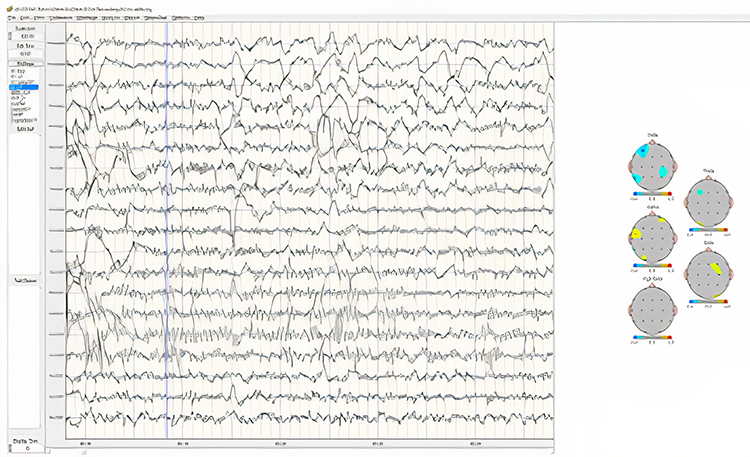
The image below shows an area of EMG artifact at T3 (jaw muscle – masseter – tension) interpreted by the program as excess high beta.
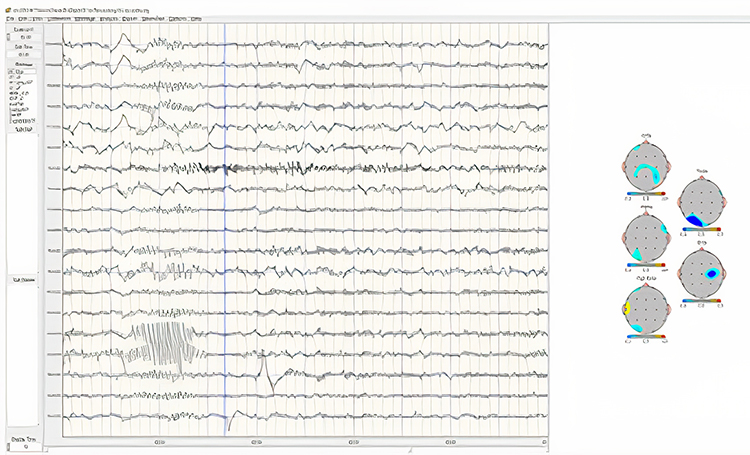
So, it is essential for the practitioner to know the system's limitations, the effect of the montage on the resulting data analysis, and how to work around or explain such variables in the written report.
Below is the first recording from this example client with areas of artifact-free data selected (pink background, red underline), avoiding areas of obvious eye movement and other artifacts. A total of 1 minute and 42 seconds was obtained from this approximately 4-minute, eyes-closed recording. This view is of the average reference montage, with the time scale (x-axis) set to 10 seconds and the voltage scale (y-axis) set to 50 uV.
This recording shows a clear posterior dominant rhythm (alpha), with the highest amplitude at O1 and O2, with symmetrical voltage in the 25-35 uV range and fairly synchronous waveforms with a frequency of approximately 10 Hz. There is also 8-9 Hz activity at T5 and T6, with higher voltage at T6. Alpha activity is also present in frontal, central, and parietal electrodes, with generally higher amplitudes on the right. There is an electrode “pop” in the T5 electrode at the 15-second mark that does not reoccur.
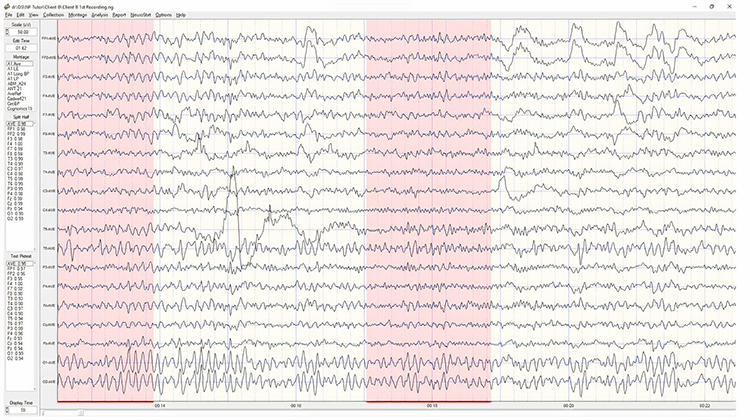
The next image shows the same time location in the longitudinal bi-polar (LongBP) montage using the same time and voltage scales. Note that the electrode pop now appears in two tracings, T3-T5 and T5-O1. Both show the large, isolated voltage discharge. The common electrode in both derivations (comparisons between two adjacent electrodes) is T5, indicating that this is the source of the voltage discharge. We can see that neither of these derivations contains this discharge when viewing F7-T3 and P3-O1 (T3 and O1 being the other electrodes in the previous derivations with T5). Therefore, we can determine that the voltage source is at T5.
Below the LongBP montage is an image of the circular bipolar montage (CircBP), which shows the discharge in the O1-T5 and T5-T3 derivations but not in the O1-O2 or the T3-F7 derivations. This same feature of the bipolar montage can be used to isolate any EEG event or activity, such as epileptiform discharges, vertex sharp waves, and other common EEG features like positive occipital sharp transients of sleep (POSTS), subclinical rhythmic epileptiform discharge of adults (SREDA) or localized delta, theta or other EEG frequency activity.
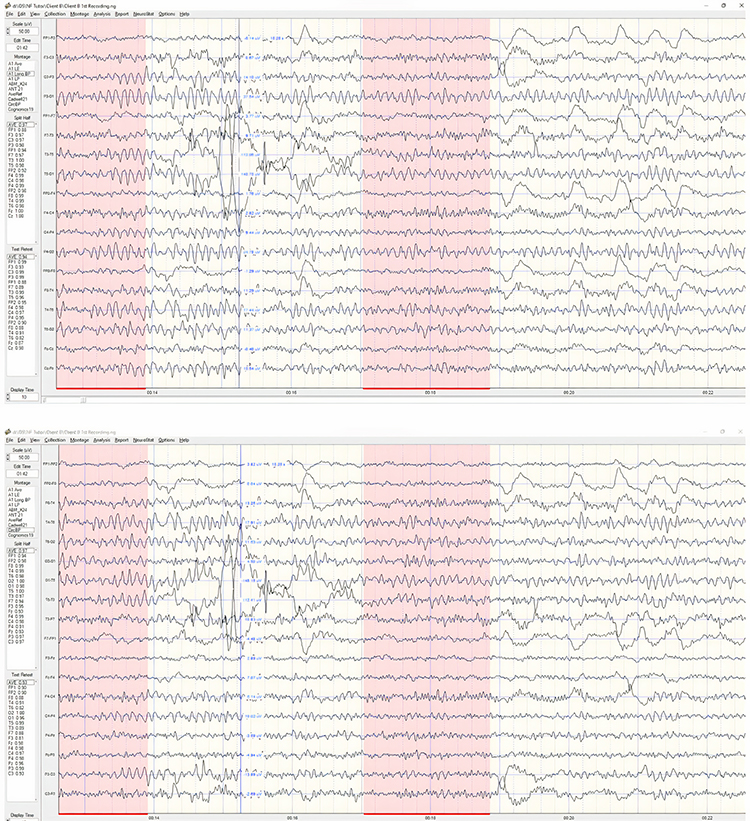
The next image shows the absolute power topographic maps in the average reference montage with each “head” image representing 1 Hz of EEG activity (called ‘bins). This is a good way to identify the peak or dominant alpha frequency by looking at the 8-12 Hz frequency band and identifying the frequency bin with the highest value. The color scale below each head shows the voltage as power (uV squared) and simply shows the voltage gradient from minimum to maximum. The head with the highest number is the peak or dominant frequency. In this case, it is 9 Hz at a power value of 67.0, representing a range from 8.5 to 9.5 Hz.
Other information derived from these absolute power maps includes the typical location of the various EEG frequencies, such as alpha - generally being of highest voltage in posterior areas – parietal and occipital primarily in the eyes-closed condition. The maximum beta activity is generally in frontal and central areas, and delta is often maximal in the frontal midline areas. In this image, 3-5 Hz is maximal at T6 and O1, suggesting a difference from the expected locations.
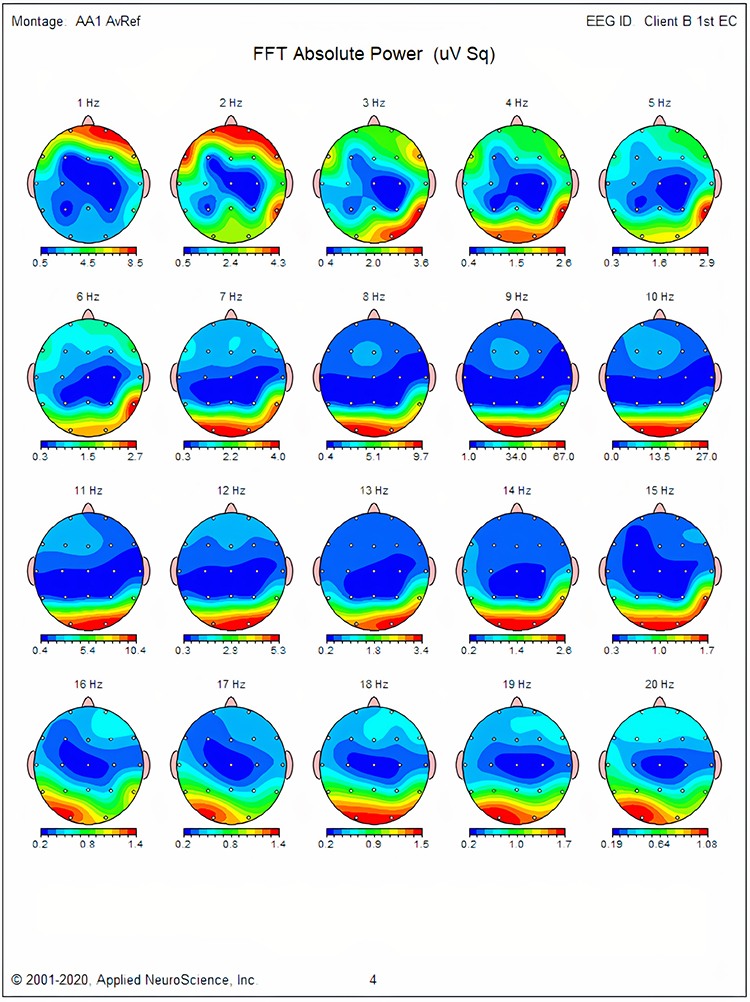
The next image shows the Z-Scored FFT Absolute Power topographic maps. In this case, the color scale under each head shows standard deviations for that frequency bin from -3 SD to +3 SD; therefore, the colors on the maps refer to deviations from expected values. There is broadly distributed excess 1 Hz activity but more localized excesses from 2-5 Hz. There is excess 9 Hz in frontal, central, and posterior areas and some excess 12-14 Hz activity in the occipital and right parietal.
We expect to see typical values for all frequency bins; therefore, deviations are of interest and can identify areas of atypical function. The somewhat localized areas of delta and theta activity may suggest areas of damage from whatever cause. The image following the one below, in the Laplacian montage, shows even more localization and may indicate a possible coup contrecoup injury sustained from an impact injury to the head from the left lateral frontal translated to the right temporal/parietal area. In fact, this client did sustain a TBI from being struck by an assailant in the left lateral frontal area. This impact appears to have caused the brain to impact the skull on the opposing side, contralateral to the original impact. This is a common type of TBI and is typically the result of being hit by a right-handed person on the left side of the head. Since approximately 90% of humans are right-handed, this is common in head-injured individuals, particularly those in physical abuse situations and those participating in boxing or full-contact martial arts. TBI from accident injuries and those from contact sports are more varied because the impacts can involve other areas of the head.
The areas showing excess 9 Hz activity are interesting. 9 Hz is a bit slow for the alpha frequency band in an adult, though it wasn’t identified as statistically significant in the peak alpha frequency table (see below). The excess in occipital and parietal areas just suggests slowing the peak frequency. The generalized frontal and central and the more localized excess focused between F3, Fz, C3, and Cz may be the result of the referencing system (common average) contributing a composite voltage to all sensors, possibly containing 9 Hz activity that is then contributed to this area as a result of common mode rejection. This activity is not as focal or localized in the Laplacian montage, which is good at removing global signals and often reveals highly localized activity.
This is an example of how the practitioner needs to be skeptical of findings that appear to present one conclusion and must instead make multiple comparisons to determine, as much as possible, what is accurate and what is not.
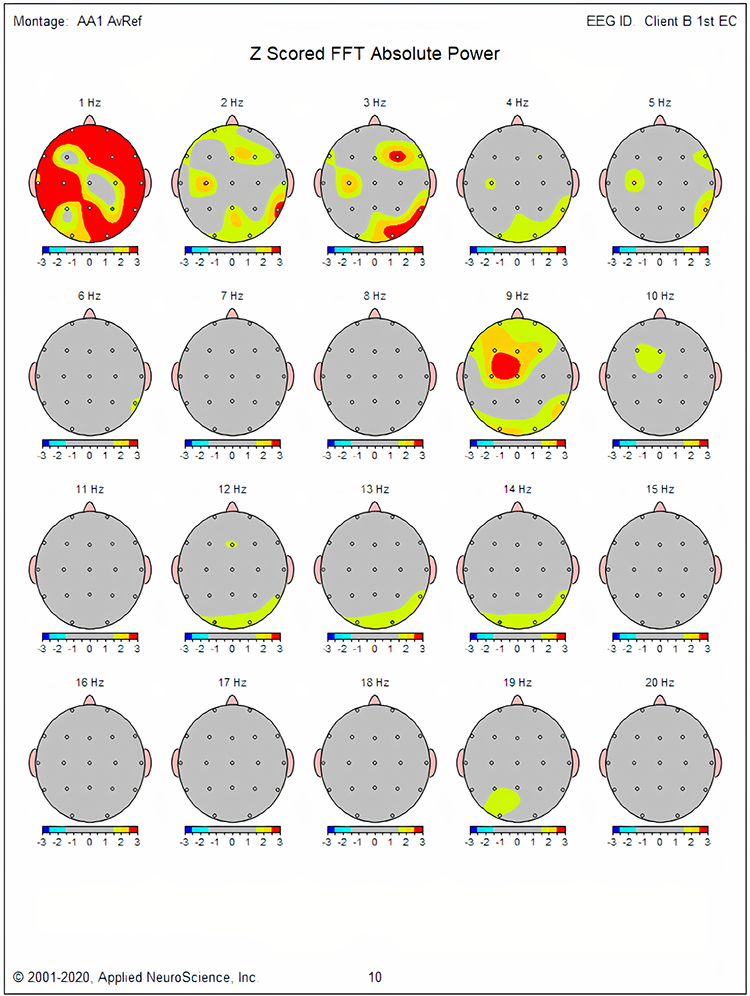
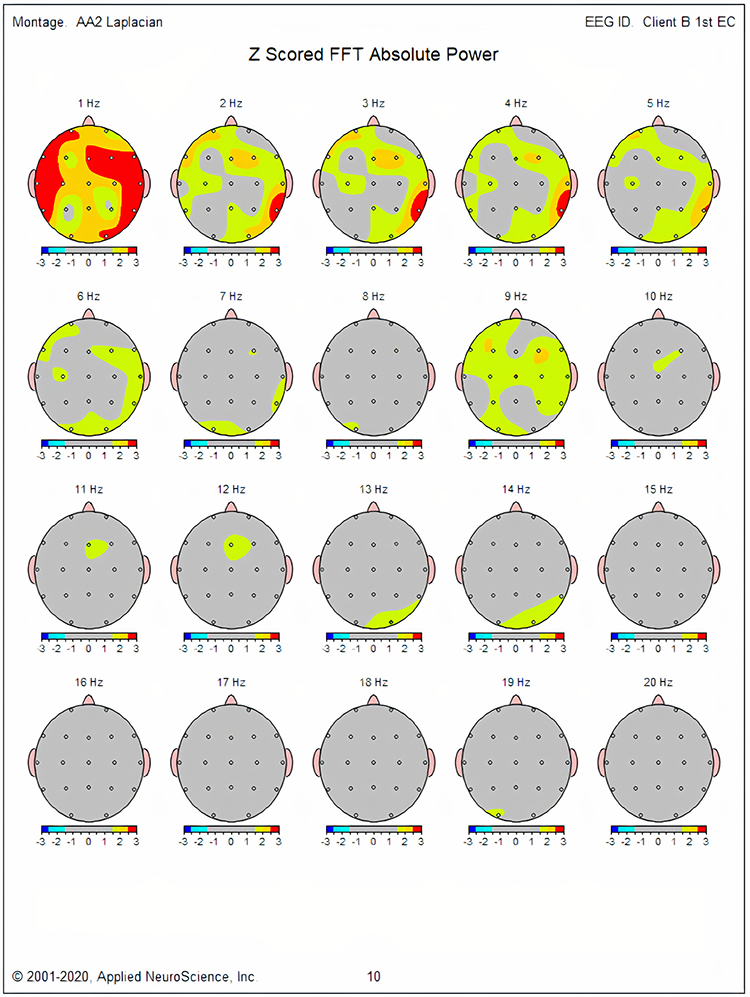
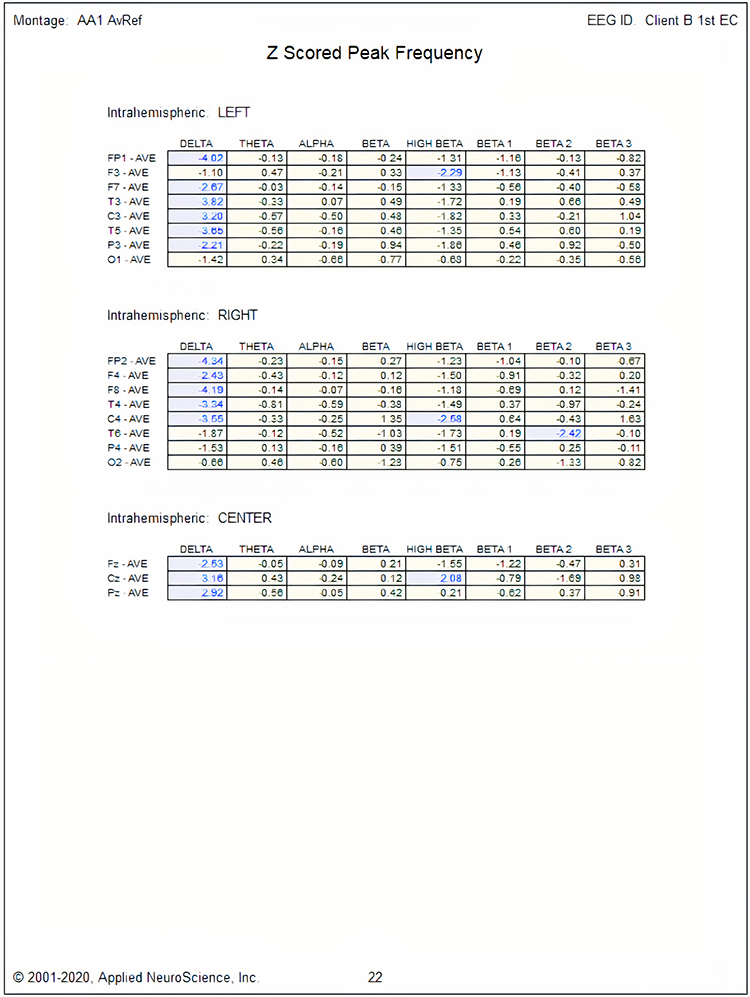
Next, we will look at the results from the linked ears montage. This is an interesting montage because it is commonly used as the source for many additional calculations such as phase, coherence, phase slope index, TBI, learning disability (LD), and other discriminant functions. Some more recent normative databases like the iSynchBrain (© 2018 iMediSync, Inc. All rights reserved) have been developed to calculate phase and coherence measures from the current density information generated by the Laplacian operator.
Earlier database approaches, such as E Roy John’s Nx-Link database and Robert Thatcher’s NeuroGuide database, use the linked ears montage. Thatcher has stated on multiple occasions that using other montages skews the phase values and, therefore, cannot be used to calculate coherence, which is a measure of the consistency of the phase relationship from one location to another.
Nunez and Srinivasan claim that if normative values are generated using the Laplacian montage, then the resulting phase and subsequent coherence calculations can be reliably used. This controversy will likely take some time and additional studies to resolve.
As we have noted previously, the linked ears montage is particularly prone to reference contamination from the linked ear or linked mastoid references because they are close to EEG sources in the temporal and parietal lobes. They then contribute to the other channels. Again, common mode rejection (CMR) is covered in depth elsewhere in qEEG Tutor. The following graphs will highlight some of these issues.
The first image below shows the client data from the previous topographic maps. Note the presence of frontal activity that wasn’t as apparent and didn’t show the same degree of deviation from normal values. An example is 9 Hz, which we have discussed previously. The linked ears montage shows 9 Hz broadly distributed across the frontal, central and temporal areas at greater than 2.5-3.0 SD. Much of this is likely due to reference contamination.
The next image shows the coherence values calculated from these linked ears data. Note that the alpha frequency band shows hypercoherence in multiple combinations with the greatest deviations in the same areas that showed excess 9 Hz. These same factors likely contribute to excess hypercoherence in other frequency bands. Unfortunately, there is no way to identify the contribution of reference contamination separate from the actual signal at those locations.
We can determine training protocols from these displays and other sources, including the client’s history and self-report. Training could begin by simply downtraining excess delta and theta activity at F7 and T4, and T6. Z-score training could also be used to train these areas toward Z=0. Nineteen-channel swLORETA Z-score training could address network functions related to the clients concerns about expressive language, particularly articulation problems and non-verbal language, particularly reading emotions. Since the F7 electrode generally resides over Broca’s area (speech articulation) and T4 and T6 generally reside over the non-verbal language/communication areas, any of these approaches will likely have positive benefits.
These are limited examples of the decision-making process used for these two clients, the first using a clinical, experiential approach and the second using the normative database approach. What isn’t directly covered is the symptom-based approach. However, both approaches described used similar decision-making processes based on an understanding of functional neuroanatomy and neurophysiology. Practitioners would do well to explore these two important topics through BioSource Software’s Functional Neuroanatomy and Physiological Psychology.
Glossary
amplitude: the strength of the EEG signal measured in microvolt or picowatts.
artifact: false signals like 50/60Hz noise produced by line current.
alpha blocking: alpha blocking normally occurs when eyes have just been opened. Arousal and specific forms of cognitive activity may reduce alpha amplitude or eliminate it while increasing EEG power in the beta range.
alpha response: increased alpha amplitude.
alpha rhythm: 8-12-Hz activity that depends on the interaction between rhythmic burst firing by a subset of thalamocortical (TC) neurons linked by gap junctions and rhythmic inhibition by widely distributed reticular nucleus neurons. Researchers have correlated the alpha rhythm with relaxed wakefulness. Alpha is the dominant rhythm in adults and is located posteriorly. The alpha rhythm may be divided into alpha 1 (8-10 Hz) and alpha 2 (10-12 Hz).
alpha spindles: trains of alpha waves that are visible in the raw EEG and are observed during drowsiness, fatigue, and meditative practice.
alpha-theta training: protocol to slow the EEG to the 6-9 Hz crossover region while maintaining alertness.
amplitude: the strength of the EEG signal measured in microvolts or picowatts, and as seen in the peak-to-trough height of EEG waves.
beta rhythm: 12-38-Hz activity associated with arousal and attention generated by brainstem mesencephalic reticular stimulation that depolarizes neurons in the thalamus and cortex. The beta rhythm can be divided into multiple ranges, often defined as: beta 1 (12-15 Hz), beta 2 (15-18 Hz), beta 3 (18-25 Hz), and beta 4 (25-38 Hz).
beta spindles: trains of spindle-like waveforms with frequencies that can be lower than 20 Hz but more often fall between 22 and 25 Hz. They may signal ADHD, especially with tantrums, anxiety, autistic spectrum disorders (ASD), epilepsy, and insomnia.
bipolar (sequential) montage: a recording method that uses two active scalp electrodes and a common reference.
clinical database approach: using information such as ratios, measures of differences from one location to another, peak frequency information, maximum voltage, minimum voltage, and other metrics to determine how the client's EEG differs from expected characteristics and what can be done to restore optimal functioning.
common-mode rejection (CMR): using a differential amplifier, eliminating simultaneous, in-phase signals that occur at the two electrode sites.
cross-frequency synchronization (CFS): a mechanism in which waves of one type of frequency, such as beta or gamma, occur synchronously with the wave patterns of slower frequencies such as delta, theta, or alpha. These are often described as nested rhythms.
default mode network (DMN): a cortical network of sites located in frontal, temporal, and parietal regions that is most active during introspection and daydreaming and relatively inactive when pursuing external goals.
delta rhythm: 0.05-3 Hz oscillations generated by thalamocortical neurons during stage 3 sleep.
depolarization: to make the membrane potential less negative by making the inside of the neuron less negative with respect to its outside.
EEG activity: electrical activity of the cortex.
EEG artifacts: noncerebral electrical activity in an EEG recording can be divided into physiological and exogenous artifacts.
electrode: a specialized conductor that converts biological signals like the EEG into currents of electrons.
frequency (Hz): the number of complete cycles that an AC signal completes in a second, usually expressed in hertz.
gamma rhythm: EEG activity frequencies above 30 or 35 Hz. Frequencies from 25-70 Hz are called low gamma, while those above 70 Hz represent high gamma.
hertz (Hz): unit of frequency measured in cycles per second.
International 10-10 system: a modified combinatorial system for electrode placement that expands the 10-20 system to 75 electrode sites to increase EEG spatial resolution and improve detection of localized evoked potentials.
International 10-20 system: a standardized procedure for 21 recording and one ground electrode on adults.
Joint Frequency Time Analysis (JFTA): an analytical procedure that plots the spectral distribution of signal power over time.
magnetoencephalography (MEG): a noninvasive functional imaging technique that uses SQUIDs (superconducting quantum interference devices) to detect the weak magnetic fields generated by neuronal activity.
magnetic resonance imaging (MRI): a noninvasive imaging technique that uses strong magnetic fields and bursts of RF energy to construct highly detailed images of the living brain.
microvolt (μV): the unit of amplitude (signal strength) that is one-millionth of a volt.
mirror neuron system: network of locations associated with what might be termed learning by observation, mimicry, or imitation.
movement artifact: voltages caused by client movement or the movement of electrode wires by other individuals.
mu rhythm: 7-11-Hz waves that resemble wickets and appear as several-second trains over central or centroparietal sites (C3 and C4).
normative database approach: comparing a client's EEG to a "normal" sample, identifying variations, and selecting training locations and frequencies.
polarization: to make the membrane potential more negative by making the inside of the neuron more negative with respect to its outside.
nystagmus: an involuntary rhythmic side-to-side, up and down, or circular motion of the eyes that occurs with a variety of conditions.
positron emission tomography (PET): a functional imaging technique that injects radioactive chemicals into the brain's circulation to measure brain activity.
posterior dominant rhythm (PDR): the highest-amplitude frequency detected at the posterior scalp when eyes are closed.
power: amplitude squared and may be expressed as microvolts squared or picowatts/resistance.
Quantitative EEG (qEEG): digitized statistical brain mapping using at least a 19-channel montage to measure EEG amplitude within specific frequency bins.
reference electrode: an electrode placed on the scalp, earlobe, or mastoid.
reticular nucleus of the thalamus (TRN): GABAergic thalamic neurons that modulate signals from other thalamic nuclei and do not project to the cortex. Also called the nucleus reticularis of the thalamus (NRT).
sensorimotor rhythm (SMR): EEG rhythm that ranges from 12-15 Hz and is located over the sensorimotor cortex (central sulcus). The waves are synchronous. The sensorimotor rhythm is associated with the inhibition of movement and reduced muscle tone. The SMR is generated by "ventrobasal relay cells in the thalamus and thalamocortical feedback loops."
single photon emission computerized tomography (SPECT): a functional imaging technique that uses gamma rays to create three-dimensional and slice images of cerebral blood flow averaged over several minutes.
slow cortical potential (SCP): slow event-related direct-current shifts of the electroencephalogram. Cortical potential shifts precede the depolarization of large cortical assemblies. The negative shift reflects the actions of gap junction cells. That shift reduces the excitation threshold of pyramidal neurons, leading to increased firing or depolarization of large assemblies of pyramidal neurons.
symptom-matching approach: a "decision tree" approach using workbooks or guides that provide an "if this, then do that" format with a series of steps to be followed if initial interventions do not provide adequate relief.
synchrony: the coordinated firing of pools of neurons due to pacemakers and mutual coordination.
thalamic-cortical relay (TCR) system: the primary pathway for determining which areas of the cortex receive each type of sensory input.
theta/beta ratio: the ratio between 4-7 Hz theta and 13-21 Hz beta, measured as amplitude squared most typically along the midline and generally in the anterior midline near the 10-20 system location Fz.
theta rhythm: 4-8-Hz rhythms generated by a cholinergic septohippocampal system that receives input from the ascending reticular formation and a noncholinergic system that originates in the entorhinal cortex, which corresponds to Brodmann areas 28 and 34 at the caudal region of the temporal lobe.
vertex (Cz): the intersection of imaginary lines drawn from the nasion to inion and between the two preauricular points in the International 10-10 and 10-20 systems.
waveform: the shape and form of an EEG signal.
TEST YOURSELF ON CLASSMARKER
Click on the ClassMarker logo below to take a 10-question exam over this entire unit.

REVIEW FLASHCARDS ON QUIZLET PLUS
Click on the Quizlet Plus logo to review our chapter flashcards.

Visit the BioSource Software Website
BioSource Software offers Functional Neuroanatomy, which reviews critical behavioral networks, and qEEG100, which provides extensive multiple-choice testing over the IQCB Blueprint.


Assignment
Now that you have completed this unit, describe how you explain the qEEG to your clients. Which training apporach do you use. Why?
References
Ancoli, S., & Kamiya, J. (1978). Methodological issues in alpha biofeedback training. Biofeedback and Self-Regulation, 3(2). 159-183. https://doi.org/0.1007/BF00998900
Arns, M., de Ridder, S., Strehl, U., Breteler, M., & Coenen, A. (2009). Efficacy of neurofeedback treatment in ADHD: The effects on inattention, impulsivity and hyperactivity: A meta-analysis. Clinical EEG and neuroscience, 40(3), 180–189. https://doi.org/10.1177/155005940904000311
Berger, H. (1929). Über das Elektrenkephalogramm des Menschen. Archiv f. Psychiatrie, 87, 527–570.
Britton, J. W., Frey, L. C., Hopp, J. L., Korb, P., Koubeissi, M. Z., Lievens, W. E., Pestana-Knight, E. M., & St. Louis. E. K., E. K. St. Louis, L. C. Frey (Eds.) (2016). Electroencephalography (EEG): An introductory text and atlas of normal and abnormal findings in adults, children, and infants. American Epilepsy Society.
Cohen, M. P. (2020). Neurofeedback 101: Rewiring the brain for ADHD, anxiety, depression and beyond (without medication). Center from Brain Training.
Cox, C. L., Huguenard, J. R., & Prince, D. A. (1997). Nucleus reticularis neurons mediate diverse inhibitory effects in thalamus. Proceedings of the National Academy of Sciences, 94(16), 8854-8859. https://doi.org/10.1073/pnas.94.16.8854
Davey, C. G., & Harrison, B. J. (2018). The brain's center of gravity: How the default mode network helps us to understand the self. World Psychiatry: Official Journal of the World Psychiatric Association (WPA), 17(3), 278-279. https://doi.org/10.1002/wps.20553
Demos, J. N. (2019). Getting started with neurofeedback (2nd ed.). W. W. Norton & Company.
Fisch, B. J. (1999). Fisch and Spehlmann's EEG primer (3rd ed.). Elsevier.
ISNR Board of Directors (2010). What is neurofeedback? Retrieved from https://isnr.org/what-is-neurofeedback.
Kamiya, J. (1961). Behavioral, subjective, and physiological aspects of drowsiness and sleep. In D. W. Fiske, & S. R. Maddi (Eds.). Functions of varied experience. Dorsey Press.
Kamiya, J. (1962). Conditioned discrimination of the EEG alpha rhythm in Humans. Abstract presented at the Western Psychological Association meeting.
Kamiya, J. (1968). Conscious control of brain waves. Psychology Today, 1, 56-63.
Kamiya, J. (1969). Operant control of the EEG alpha rhythm and some of its reported effects on consciousness. In C. Tart (Ed.), Altered states of consciousness. John Wiley and Sons.
Nicholson, A. A., Densmore, M., MicKinnon, M. C., Neufeld, R. W. J., Frewen, P. A. Théberge, J., Jetly, R., Richardson, J. D., & Lanius, R. A. (2019). Machine learning multivariate pattern analysis predicts classification of posttraumatic stress disorder and its dissociative subtype: A multimodal neuroimaging approach. Psychol Med, 49(12), 2049-2059. https://doi.org/10.1017/S003329171800286
Nicholson, A. A., Ros, T., Densmore, M., Frewen, P. A., Neufeld, R. W. J., Théberge, J., Jetly, R., & Lanius, R. A. (2020). A randomized, controlled trial of alpha-rhythm EEG neurofeedback in posttraumatic stress disorder: A preliminary investigation showing evidence of decreased PTSD symptoms and restored default mode and salience network connectivity using fMRI. Neuroimage Clin., 28, 102490. https://doi.org/10.1016/j.nicl.2020.102490
Nicholson, A. A., Ros, T., Jetly, R., & Lanius, R. (2020). Regulating posttraumatic stress disorder symptoms with neurofeedback: Regaining control of the mind. Journal of Military Veteran and Family Health 6(S1), 3-15. https://doi.org/10.3138/jmvfh.2019-0032
Othmer, S. (2006). Protocol guide for neurofeedback clinicians. EEG Info, Inc.
Peniston, E. G., & Kulkosky, P. J. (1989). Alpha-theta brain wave training and beta-endorphin levels in alcoholics. Alcoholism, Clinical and Experimental Research, 13, 271–279. https://doi.org/10.1111/j.1530-0277.1989.tb00325.x
Peniston, E. G., & Kulkosky, P. J. (1990). Alcoholic personality and alpha-theta brainwave training. Medical Psychotherapy, 3, 37–55.
Pfister, H., Kaynig, V., Botha, C. P., Bruckner, S., Dercksen, V., & Hege, H.-C. (2012). Visualization in connectomics. Mathematics and Visualization, 37. https://doi.org/10.1007/978-1-4471-6497-5_21
Scott, W., & Kaiser, D. (2005). Effects of an EEG biofeedback protocol on a mixed substance abusing population. The American Journal of Drug and Alcohol Abuse, 31(3), 455-469. https://doi.org/10.1081/ada-200056807
Swingle, P. G. (2008). Biofeedback for the brain: How neurotherapy effectively treats depression, ADHD, autism, and more. Rutgers University Press.
Swingle, P. G. (2015). Adding neurotherapy to your practice: Clinician's guide to the ClinicalQ, neurofeedback, and braindriving. Springer International Publishing. https://doi.org/10.1007/978-3-319-15527-2
Thompson, M., & Thompson, L. (2015). The biofeedback book: An introduction to basic concepts in applied psychophysiology (2nd ed.). Association for Applied Psychophysiology and Biofeedback.
Weise, R., von Mengden, I., Glos, M., Garcia, C., & Penzel, T. (2013). P 153. Influence of transcranial slow oscillation current stimulation (tSOS) on EEG, sleepiness and alertness. Clinical Neurophysiology, 124(10), e137. https://doi.org/10.1016/j.clinph.2013.04.230
